CBSE Class 11-science Answered
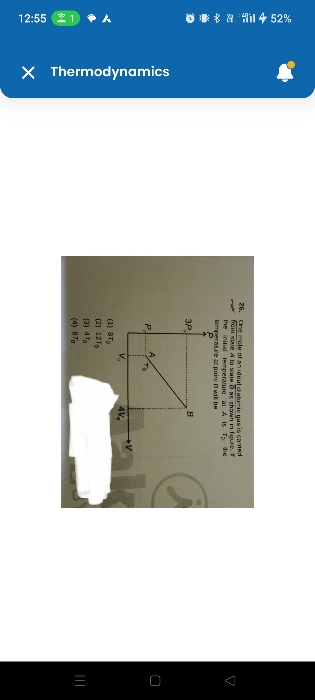
Maximum efficiency of an engine working between temperatures T2 and T1 is given by the fraction of the heat absorbed by an engine which can be converted into work is known as efficiency of the heat engine.
Mathematically,
Efficiency, ? = (T2 T1) / T2 = 1/5
5 T2 -5 T1 = T2
Therefore, T2 = 1.25 T1
.. (1)
Where T2 is the source temperature and T1 is the sink temperature.
If the sink temperature (T1) is reduced by 70o C, the efficiency is doubled
?= T2 (T1 70) / T2 = 2x1/5 = 2/5
5 T2 5 T1 + 350 = 2 T2
5 T2 2 T2 5 T1 + 350 = 0
3T2 5 T1 + 350 = 0
Substituting the value of T2 from equation (1), we will get,
3(1.25 T1) 5 T1 + 350 = 0
3.75T1 5 T1 + 350 = 0
350 = 1.25 T1
T1 = 300 K
Therefore, T2 = 1.25 X 300 = 375 K
Hence, temperature of the source and the sink is 375 K and 300 K respectively.