CBSE Class 11-science Answered
A narrow cylindrical tube,80 cm long and open at both ends is half immersed in Mercury. Then the top of the tube is closed and it is taken out of Mercury A column of Mercury 22 cm long remains in the tube The atmospheric pressure is
Asked by nagalakshmisastry | 18 Sep, 2019, 12:33: AM
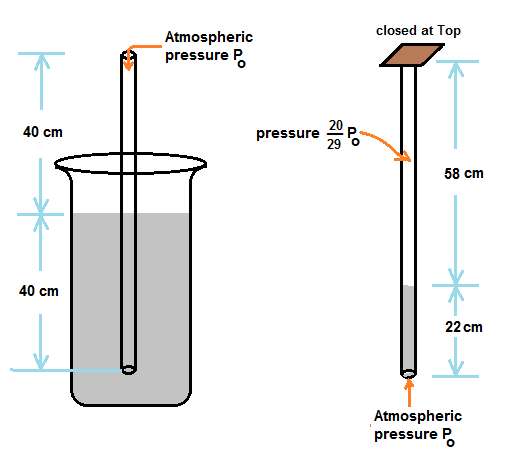
When half of tube is immersed in mercury, pressure of air inside other half of tube above mercury is atmospheric pressure Po ,
When top of tube is closed by hand and removed from mercury, it is given that tube is holding mercury of 22 cm tube length.
Hence trapped air volume above mercury in tube is 58A , where A is cross section area of tube.
Since the original volume of air inside tube is 40A, pressure of trapped air after closing the top of tube is Po(40A/58A) = (20/29)Po
Now trapped air pressure and pressure due to 22 cm of mercury together balance the atmospheric pressure
Hence we have, (20/29)Po + 22 cm of mercury pressure = Po
22 cm of mercury pressure = (20/29) Po or atmospheric pressure Po = 70.9 cm of Mercury pressure
Answered by Thiyagarajan K | 18 Sep, 2019, 05:13: PM
CBSE 11-science - Physics
Asked by sheikhsaadat24 | 17 Apr, 2024, 09:41: PM
CBSE 11-science - Physics
Asked by sy123946 | 07 Apr, 2024, 04:23: PM
CBSE 11-science - Physics
Asked by derhebha955 | 03 Apr, 2024, 09:03: AM
CBSE 11-science - Physics
Asked by sumedhasingh238 | 29 Mar, 2024, 05:15: PM
CBSE 11-science - Physics
Asked by sumedhasingh238 | 28 Mar, 2024, 11:10: PM
CBSE 11-science - Physics
Asked by roshnibudhrani88 | 23 Mar, 2024, 05:52: PM
CBSE 11-science - Physics
Asked by emad.amd | 21 Mar, 2024, 12:00: PM
CBSE 11-science - Physics
Asked by vinitdubey7735 | 14 Mar, 2024, 11:21: AM
CBSE 11-science - Physics
Asked by om636694 | 04 Mar, 2024, 09:10: PM
CBSE 11-science - Physics
Asked by rajuinwati12 | 04 Mar, 2024, 09:22: AM




