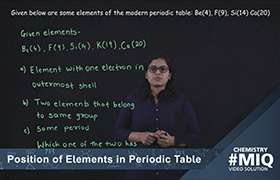
CBSE Class 10 Answered
(a) Lithium, sodium, potassium are all metals that react with water to liberate hydrogen gas. Is there any similarity in the atoms of these elements? If yes, write the similarity.
(b) Helium is a non-reactive gas and neon is a gas of extremely low reactivity. What, if anything, do their atoms have in common?
Asked by Topperlearning User | 27 May, 2014, 09:49: AM
(a) Yes, the atoms of all the three elements lithium, sodium and potassium have one electron each in their outermost shells.
(b) Both helium (He) and neon (Ne) have filled outermost shells. Helium has a duplet in its K shell, while neon has an octet in its L shell.
Answered by | 27 May, 2014, 11:49: AM
Application Videos
Concept Videos
CBSE 10 - Chemistry
Asked by dhumalchhaya13 | 26 May, 2022, 11:05: PM
CBSE 10 - Chemistry
Asked by kanchanbalpande82 | 12 Apr, 2022, 11:01: AM
CBSE 10 - Chemistry
Asked by kanchanbalpande82 | 12 Apr, 2022, 11:01: AM
CBSE 10 - Chemistry
Asked by waghmaresheetal78 | 27 Dec, 2021, 05:02: PM
CBSE 10 - Chemistry
Asked by ksheera36 | 03 Jun, 2021, 08:35: PM
CBSE 10 - Chemistry
Asked by vungtsaniyanthan | 16 May, 2021, 06:32: PM
CBSE 10 - Chemistry
Asked by sinhagopalakumara | 01 May, 2021, 08:16: PM
CBSE 10 - Chemistry
Asked by advssdrall | 26 Mar, 2021, 07:43: AM
CBSE 10 - Chemistry
Asked by shettyshrinidhi271 | 07 Jan, 2021, 05:04: PM
CBSE 10 - Chemistry
Asked by adipadmakarri | 05 Dec, 2020, 06:41: PM