CBSE Class 9 Answered
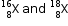 in the sample?
(b) Complete the following table.
Elements
Atomic Number
Mass Number
Protons
Neutrons
Electrons
A
11
-
-
12
-
B
-
35
-
-
17
in the sample?
(b) Complete the following table.
Elements
Atomic Number
Mass Number
Protons
Neutrons
Electrons
A
11
-
-
12
-
B
-
35
-
-
17(a) The arrangement of electrons in different orbits around the nucleus in an atom is called the electronic configuration of the element.
The following general rules used to write the electronic configuration of an element are:
(i) The shells of energy levels are represented by the circles. Electrons are filled in order of increasing energy levels of shells. e.g. first K, then L, then M and so on.
(ii) The maximum number if electrons which can be accommodated in any shell is '2n2' where 'n' is the shell number.
(iii) The outermost shell cannot accommodate more than 8 electrons, even if it has the capacity to accommodate more electrons.
(b)Atomic number = 11
Mass number = 23
Hence,
Number of protons = 11
number of electrons = 11
Mass number = P + N
23 = 11 + N
N = 23 - 11 = 12
OR
(a)Atomic mass of element X = 16.2 u
Let the % of isotope 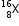 be Z.
be Z.
Let the % of isotope 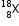 be (100 - Z).
be (100 - Z).
Hence,
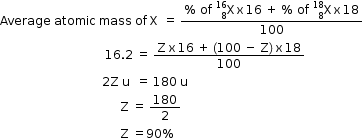
So, % of 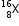 = 90%
= 90%
% of 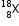 = 10%
= 10%
|
Elements |
Atomic Number |
Mass Number |
Protons |
Neutrons |
Electrons |
|
A |
11 |
23 |
11 |
12 |
11 |
|
B |
17 |
35 |
17 |
18 |
17 |









