A DIATOMIC MOLECULE IS MADE OF TWO MASSES m1 and m2 WHICH ARE SEPERATED BY DISTANCE r. calculate its enrgy by applying bohrs rule OF ANGULAR MOMEMETUM QUANTINIZATION ITS ENERGY WILL BE
PLS GIVE THE answer
- Here we need to find the moment of inertia due to both the masses m1 and m2.
- For m1 radius can be given as
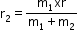 and for m2 radius can be given as
and for m2 radius can be given as 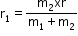
- Angular momentum is given as
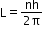 and two moments of inertias I1 and I2 can be added to get I1+I2=I
and two moments of inertias I1 and I2 can be added to get I1+I2=I - Rotational energy can be calculated from
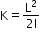
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
You have rated this answer /10
Browse free questions and answers by Chapters
- 1 Thermodynamics
- 2 Gravitation
- 3 Electromagnetic Waves
- 4 Communication Systems
- 5 Laws of Motion
- 6 Current Electricity
- 7 Work, Energy and Power
- 8 Kinematics
- 9 Physics and Measurement
- 10 Rotational Motion
- 11 Properties of Solids and Liquids
- 12 Kinetic Theory of Gases
- 13 Oscillations and Waves
- 14 Electrostatics
- 15 Magnetic Effects of Current and Magnetism
- 16 Electromagnetic Induction and Alternating Currents
- 17 Optics
- 18 Dual Nature of Matter and Radiation
- 19 Atoms and Nuclei
- 20 Electronic Devices
