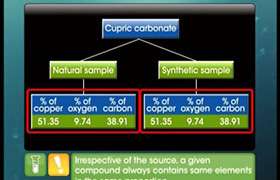
CBSE Class 11-science Answered
A compound contains carbon,hydrogen and oxygen have 40% carbon . If it's empirical formula mass = 30 amusing then what will the empirical formula of compound?
Asked by shivamguvrele15 | 18 Jun, 2019, 08:41: AM
Given:
C = 40%
Empirical formula mass = 30.
So mas of carbon =
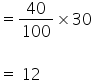
Mass of remaining elements = 30 - 12
Mass of O + H = 18
Therefore, there is one oxygen and two hydrogen atoms.
So the empirical formula of compound is CH2O.
Answered by Varsha | 18 Jun, 2019, 11:55: AM
Concept Videos
CBSE 11-science - Chemistry
Asked by dhrubapratimc | 13 Sep, 2023, 09:34: PM
CBSE 11-science - Chemistry
Asked by abhiabhishek842006 | 09 Jan, 2023, 08:34: PM
CBSE 11-science - Chemistry
Asked by Shashisinghkusum | 13 Jul, 2022, 02:44: PM
CBSE 11-science - Chemistry
Asked by shivanshiarora3457 | 09 Jun, 2022, 03:28: PM
CBSE 11-science - Chemistry
Asked by rishamariyam222 | 28 Sep, 2021, 09:18: PM
CBSE 11-science - Chemistry
Asked by seeni2005 | 08 Feb, 2021, 10:52: AM
CBSE 11-science - Chemistry
Asked by manteaditya8 | 08 Jan, 2021, 01:45: PM
CBSE 11-science - Chemistry
Asked by muanputhomte4 | 11 Nov, 2020, 09:01: PM
CBSE 11-science - Chemistry
Asked by pradeepprince858 | 07 Aug, 2020, 12:54: PM
CBSE 11-science - Chemistry
Asked by seeni2005 | 28 Jul, 2020, 11:03: AM