ICSE Class 9 Answered
a balloon speed with hydrogen at room temperature it will burnt if pressure exceed 0.2bar if at 1bar pressure the gas occupies 2.17 volume upto.what wil the volume of balloon be expanded?
Asked by indudhiman05 | 27 Aug, 2018, 09:19: PM
Given:
P1 = 1 bar
= 0.986 atm
V1 = 2.17 litre
P2 = 0.2 bar
= 0.1973
V2 = ?
According to Boyle's law;
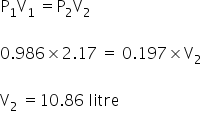
Volume of gas will be 10.86 litres.
Answered by Varsha | 28 Aug, 2018, 12:28: PM
Concept Videos
ICSE 9 - Chemistry
Asked by zainaali39692 | 04 Dec, 2020, 08:53: AM
ICSE 9 - Chemistry
Asked by gup.navya2006 | 01 Dec, 2020, 09:28: AM
ICSE 9 - Chemistry
Asked by Vishusingh2020.2021 | 25 Sep, 2020, 10:09: PM
ICSE 9 - Chemistry
Asked by sudesghnapattanayak2017 | 19 May, 2020, 08:13: PM
ICSE 9 - Chemistry
Asked by abeshchakraborty6 | 23 Feb, 2020, 08:54: AM
ICSE 9 - Chemistry
Asked by dnlwalkers | 08 Jan, 2020, 09:57: AM
ICSE 9 - Chemistry
Asked by raichuratanvi | 14 Dec, 2019, 12:02: PM
ICSE 9 - Chemistry
Asked by merajanjum87 | 21 Nov, 2019, 09:53: PM
ICSE 9 - Chemistry
Asked by parvathimanjunath24 | 31 Oct, 2019, 09:34: PM
ICSE 9 - Chemistry
Asked by devbhaisha.tl | 21 Sep, 2019, 10:02: PM



