A 5 litre flask contain hundred gram of Sulphur trioxide and one gram of helium at 20 degree Celsius. calculate the partial pressure of sulphur trioxide and helium and also total pressure?
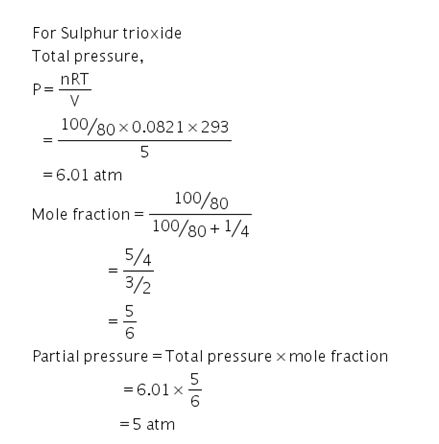
For Helium to calculate the total pressure and partial pressure you can use the same formula and steps as givan above.
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
You have rated this answer /10
Browse free questions and answers by Chapters
- 1 Classification of Elements and Periodicity in Properties
- 2 Chemical Bonding and Molecular Structure
- 3 States of Matter
- 4 Equilibrium
- 5 Hydrogen
- 6 Hydrocarbons
- 7 Environmental Chemistry
- 8 Solutions
- 9 Chemical Kinetics
- 10 Surface Chemistry
- 11 Biomolecules
- 12 Polymers
- 13 Chemistry in Everyday Life
- 14 Atomic Structure
- 15 Chemical Thermodynamics
- 16 Redox Reactions and Electrochemistry
- 17 p-Block Elements
- 18 d - and f - Block Elements
- 19 Some Basic Principles of Organic Chemistry
- 20 Organic Compounds Containing Halogens
- 21 Organic Compounds Containing Oxygen
- 22 Organic Compounds Containing Nitrogen
- 23 Co-ordination Compounds
- 24 Purification and Characterisation of Organic Compounds
- 25 s-Block Element (Alkali and Alkaline Earth Metals)
- 26 Solid State
- 27 Some Basic Concepts in Chemistry
- 28 General Principles and Processes of Isolation of Metals
- 29 Principles Related to Practical Chemistry
