CBSE Class 12-science Answered
A 0.01 solution of K3(Fe(CN)6) is 50% dissociated at 27degrees then the osmotic pressure of the solution will be
1. 0.02atm
2. 0.61atm
3. 0.78atm
4. 1.29atm
Asked by damarlasumanjali | 20 Apr, 2015, 06:25: PM
Let the degree of dissociation be 'a' then
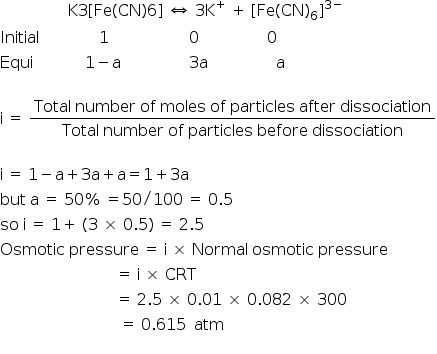
Answered by | 20 Apr, 2015, 07:09: PM
Application Videos
Concept Videos
CBSE 12-science - Chemistry
Asked by hannamaryphilip | 17 Apr, 2024, 11:20: PM
CBSE 12-science - Chemistry
Asked by ukg8612 | 15 Apr, 2024, 07:36: PM
CBSE 12-science - Chemistry
Asked by sameerteli003 | 08 Apr, 2024, 11:48: PM
CBSE 12-science - Chemistry
Asked by navadeepnavadeep242 | 19 Mar, 2024, 08:56: PM
CBSE 12-science - Chemistry
Asked by kavitabawane190 | 08 Mar, 2024, 05:24: PM
CBSE 12-science - Chemistry
Asked by hanihope27 | 01 Mar, 2024, 08:33: PM
CBSE 12-science - Chemistry
Asked by rashmij34 | 27 Feb, 2024, 04:42: PM
CBSE 12-science - Chemistry
Asked by anubhutiupadhaya | 27 Feb, 2024, 04:28: PM
CBSE 12-science - Chemistry
Asked by sagarmishra | 27 Feb, 2024, 04:01: PM
CBSE 12-science - Chemistry
Asked by yashwanthgowdakn4 | 22 Feb, 2024, 09:14: PM












