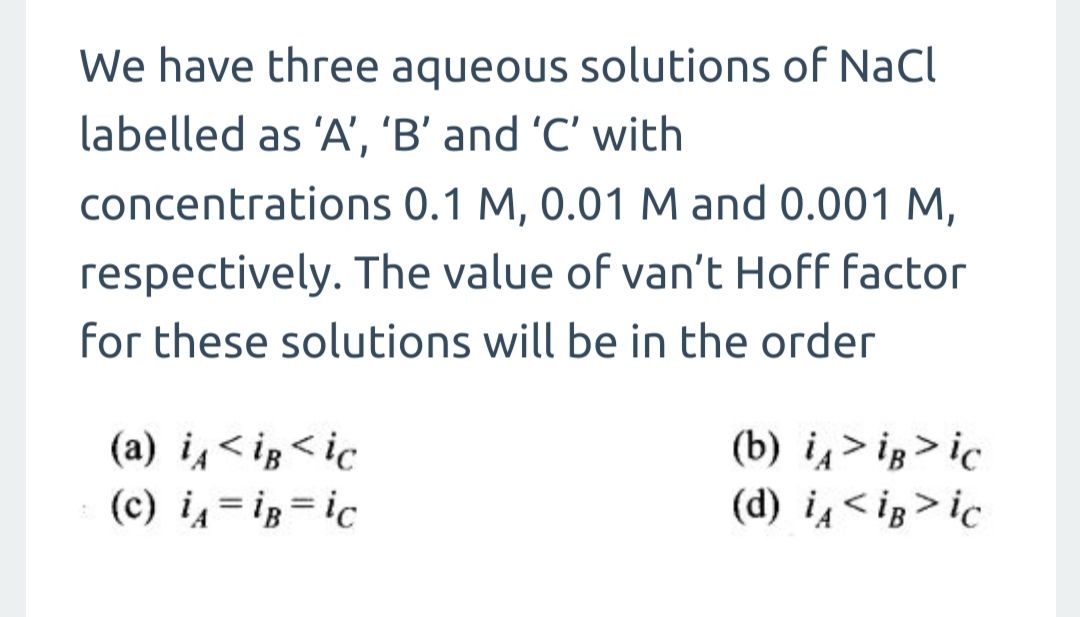
CBSE Class 12-science Answered
What does the line, ' Depending on the vapour pressures of the pure components 1 and 2, total vapour pressure over the solution decreases or increases with the increase of the mole fraction of component 1' ?
Asked by banikratul8 | 21 Mar, 2016, 01:35: AM
Total vapour pressure over the solution can be related to the mole fractions of any one component. Total vapour pressure over the solution varies linearly with the mole fraction of component 2. Therefore, depending on the vapour pressures of the pure components 1 and 2, total vapour pressure over the solution decreases or increases with the increase of the mole fraction of component 1. It is clearly explained by the plot given below.
The above plot shows that the plot of p1 or p2 verses the mole fractions x1 & x2 for a solution is linear hence p1 & p2 are directly proportional to x1 & x2 respectively. In Figure, the dashed lines I & II represent the partial pressures of components, while the marked Line III represents the total vapour pressure of the solution.
Answered by Hanisha Vyas | 21 Mar, 2016, 01:27: PM
Concept Videos
CBSE 12-science - Chemistry
Asked by kalandi.charan.407 | 08 Feb, 2024, 01:42: PM
CBSE 12-science - Chemistry
Asked by RAJAGUPTA | 01 Jan, 2020, 08:19: PM
CBSE 12-science - Chemistry
Asked by patra04011965 | 18 Jul, 2019, 04:07: PM
CBSE 12-science - Chemistry
Asked by govtsecschoolnayaganv051 | 16 Jun, 2019, 10:55: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 20 Jun, 2016, 03:50: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM