ICSE Class 10 Answered
Select the correct opttion
1 Hydroxide sparingly soluble in water is
Mgoh2
Feoh2
Caoh2
2 When aamonium chloride is heated it undergoes
Thermal decomposition or thermal dolissociation
Asked by lovemaan5500 | 20 Jan, 2018, 05:50: PM
1.
All the three hydroxides are sparingly soluble in water.
But in case of magnesium hydroxide, only 6.4 milligrams of Mg (OH)2 will dissolve in 100 ml of water.
Therefore it can be consider as insoluble in water.
2.
Ammonium chloride undergoes thermal dissociation on heating, to give ammonia and hydrogen chloride.
Ammonium chloride is again form as ammonia and hydrogen chloride will recombine at the cooler part,
that refers to the only change of state (sublimation) and not decomposition.
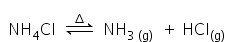
Answered by Varsha | 22 Jan, 2018, 01:08: PM
Concept Videos
ICSE 10 - Chemistry
Asked by tk5363508 | 01 Apr, 2024, 08:36: PM
ICSE 10 - Chemistry
Asked by dhakankar | 25 Oct, 2023, 11:07: AM
ICSE 10 - Chemistry
Asked by suhani.goyal.brps | 19 Oct, 2020, 08:17: PM
ICSE 10 - Chemistry
Asked by praptithombre47.10spicertl | 13 Jun, 2020, 01:09: PM
ICSE 10 - Chemistry
Asked by kartikmangaliya22.10spicertl | 27 May, 2020, 10:01: PM
ICSE 10 - Chemistry
Asked by vsejwani53 | 01 Jan, 2020, 12:23: PM
ICSE 10 - Chemistry
Asked by pradyumna2008civil | 10 Nov, 2019, 12:05: PM
ICSE 10 - Chemistry
Asked by mamtapramod1976 | 27 Sep, 2019, 06:19: PM
ICSE 10 - Chemistry
Asked by pradipdhole | 09 Jun, 2019, 11:03: PM
ICSE 10 - Chemistry
Asked by shreya.saraf.2005 | 15 Feb, 2019, 03:30: PM




