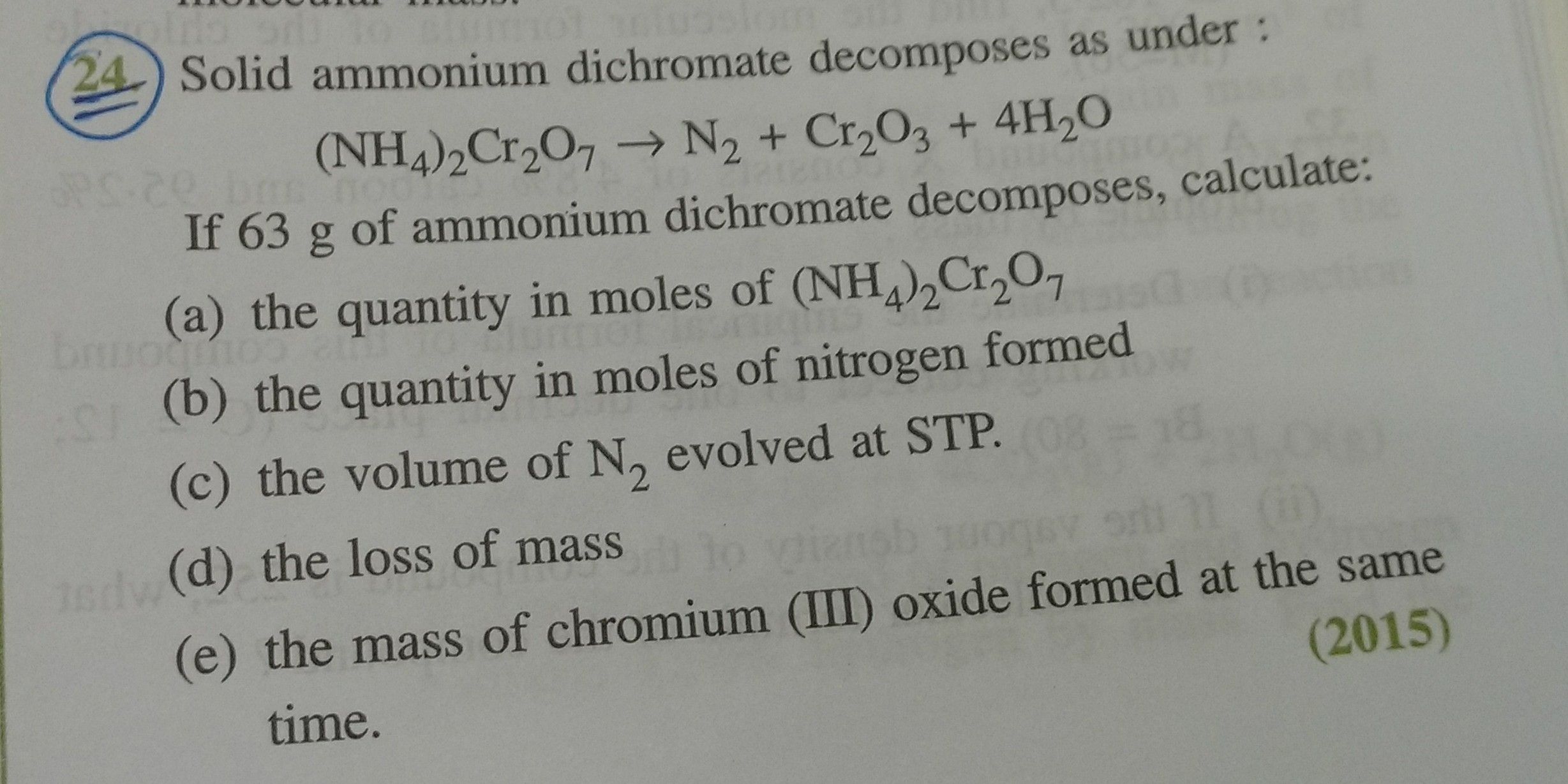ICSE Class 10 Answered
Please provide detailed steps :
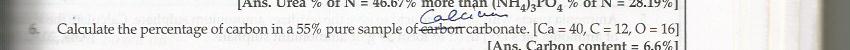
Asked by rickykumar00001 | 19 Feb, 2016, 08:08: PM
Molar mass of CaCO3 = 100 g (40+12+48)
In 100 g CaCO3, 12 g Carbon is present.
That means 12 % carbon is present in CaCO3.
So in 55% pure sample, 55x12/100 = 6.6% carbon is present.
Answered by Prachi Sawant | 21 Feb, 2016, 02:34: PM
Application Videos
Concept Videos
ICSE 10 - Chemistry
Asked by maybe.kushagra | 25 Jan, 2024, 03:12: AM
ICSE 10 - Chemistry
Asked by srinu2020.ravipati | 16 Sep, 2020, 03:33: PM
ICSE 10 - Chemistry
Asked by Gurdev71 | 24 Jun, 2020, 12:41: PM
ICSE 10 - Chemistry
Asked by Kanwaranita10 | 16 Feb, 2020, 11:22: AM
ICSE 10 - Chemistry
Asked by aashimegh | 17 Aug, 2019, 02:24: PM
ICSE 10 - Chemistry
Asked by aashimegh | 03 Aug, 2019, 11:50: AM
ICSE 10 - Chemistry
Asked by Shrinivasdangi07 | 21 Mar, 2019, 10:34: PM
ICSE 10 - Chemistry
Asked by johncena9384 | 26 Oct, 2018, 04:08: PM
ICSE 10 - Chemistry
Asked by yajay0441 | 27 Aug, 2018, 03:15: PM