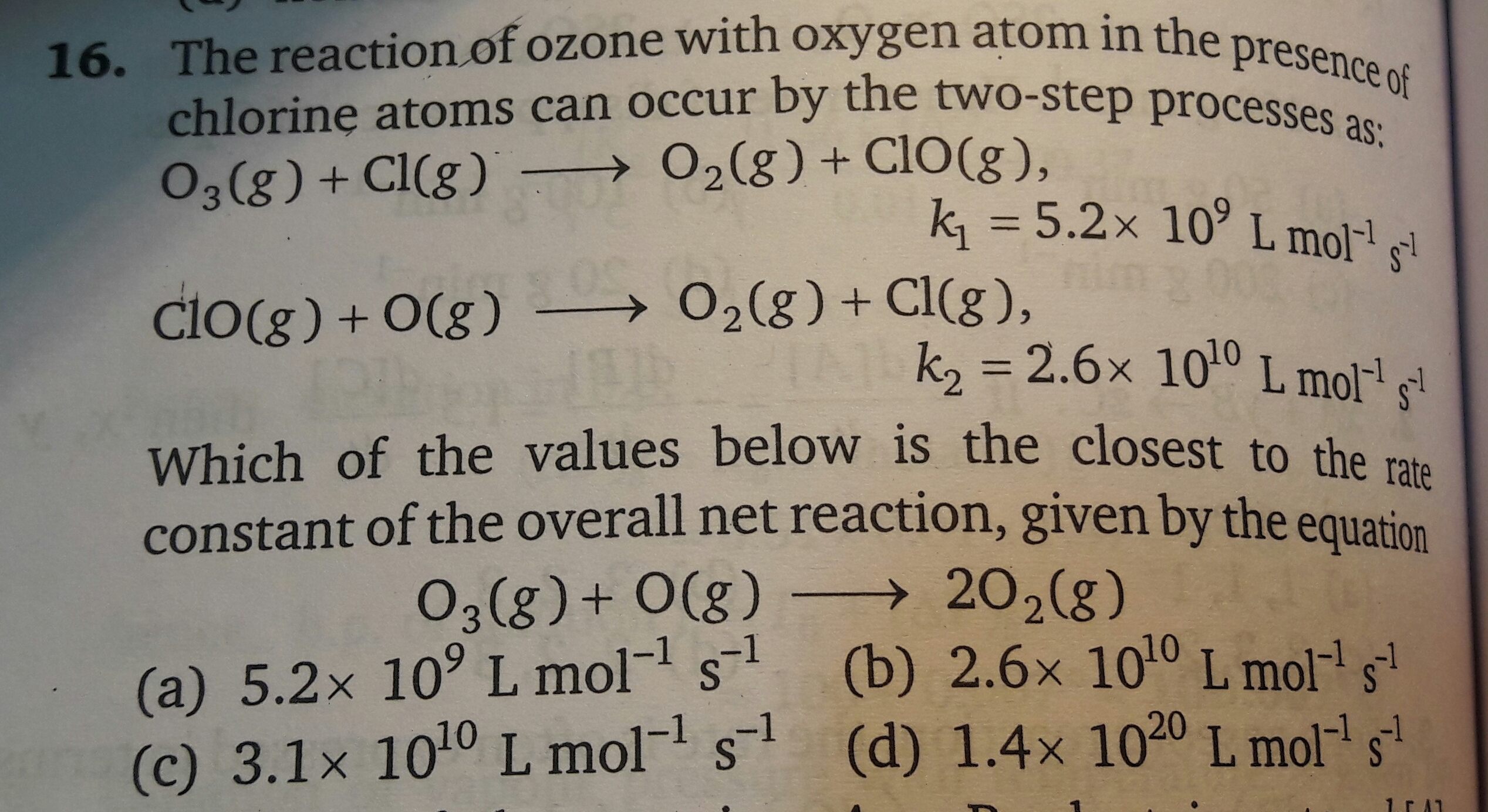 Please explain answer with calculation. Thanks. Also which formula is to be used in these kind of questions? Please give examples.
Please explain answer with calculation. Thanks. Also which formula is to be used in these kind of questions? Please give examples.
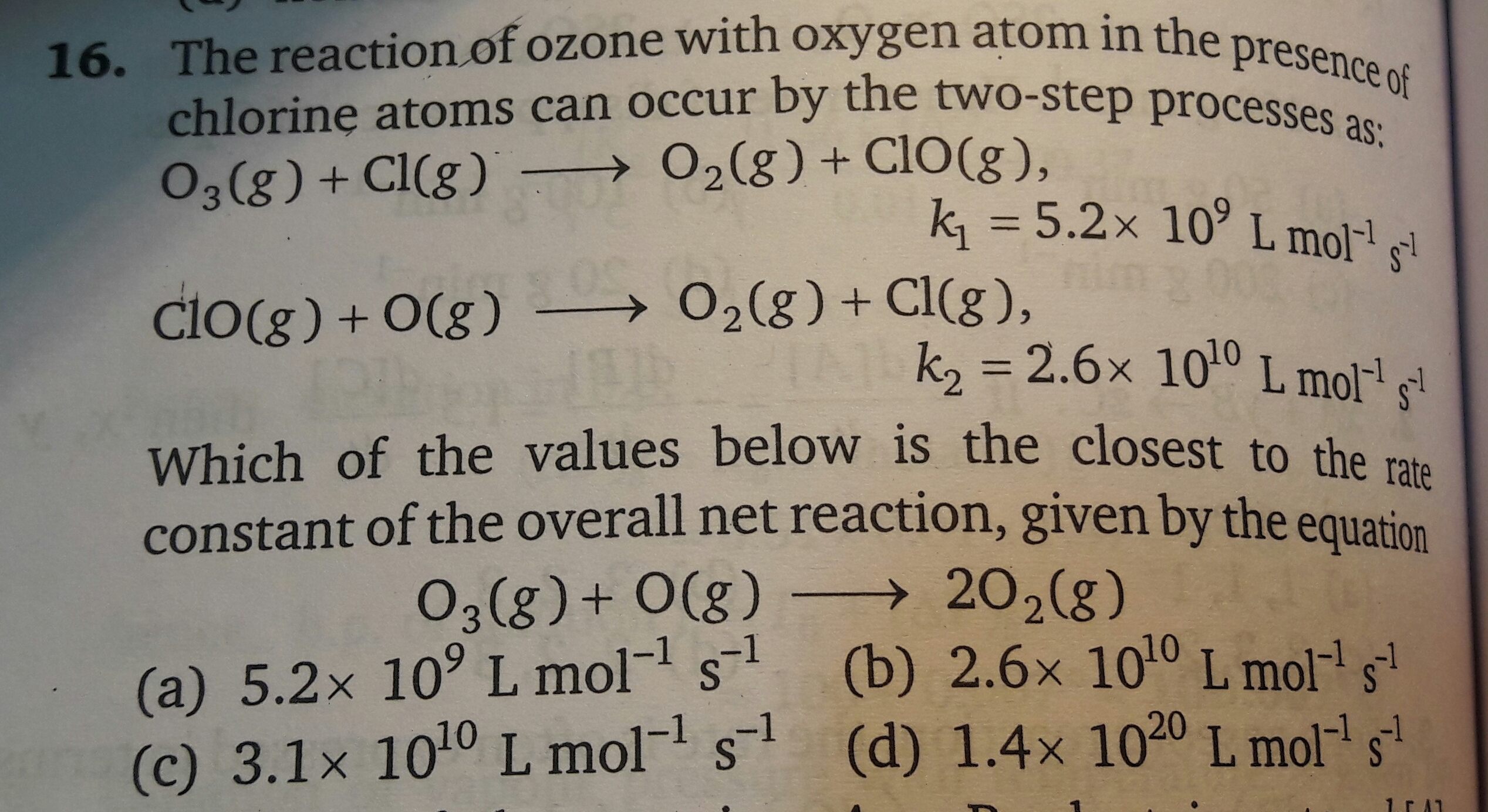 Please explain answer with calculation. Thanks. Also which formula is to be used in these kind of questions? Please give examples.
Please explain answer with calculation. Thanks. Also which formula is to be used in these kind of questions? Please give examples.In this reaction, the first step is the rate-limiting step. No matter how fast the second step takes place, the overall reaction cannot proceeds any faster than the first step in the reaction. The rate constant for the first reaction is given 5.2 × 109 L / mol.s. So closest value to the rate constant to the overall net reaction will be 5.2 × 109 L / mol.s.
Regards
Topperlearning Team.
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
You have rated this answer /10
