NEET Class neet Answered
75% of a zero order reaction complete in 4 hour ,87.5% of the same reaction complete in
Asked by mdy746807 | 25 Dec, 2020, 11:41: AM
Using the relation,
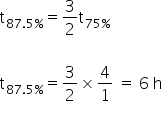
Answered by Ramandeep | 29 Dec, 2020, 05:34: PM
Application Videos
Concept Videos
NEET neet - Chemistry
Asked by mahendar160786 | 16 Apr, 2024, 09:23: PM
NEET neet - Chemistry
Asked by raomayankup83 | 15 Apr, 2024, 07:46: PM
NEET neet - Chemistry
Asked by muskannawab11 | 14 Apr, 2024, 03:13: PM
NEET neet - Chemistry
Asked by 8239682116rahul | 10 Apr, 2024, 01:48: PM
NEET neet - Chemistry
Asked by tarasingrathod63 | 07 Apr, 2024, 01:07: PM
NEET neet - Chemistry
Asked by fathimahusna6122 | 05 Apr, 2024, 10:25: AM
NEET neet - Chemistry
Asked by vasantagomasi23 | 05 Apr, 2024, 08:35: AM
NEET neet - Chemistry
Asked by ankuruthanuriya | 03 Apr, 2024, 10:56: PM
NEET neet - Chemistry
Asked by ghousiakaneez | 03 Apr, 2024, 12:55: PM




















