CBSE Class 11-science Answered
6.02 * 10ki power 20 molecules of urea are presentin 100ml of solution the concentration of urea solution is
Asked by puneetgarg2555 | 22 May, 2018, 07:16: AM
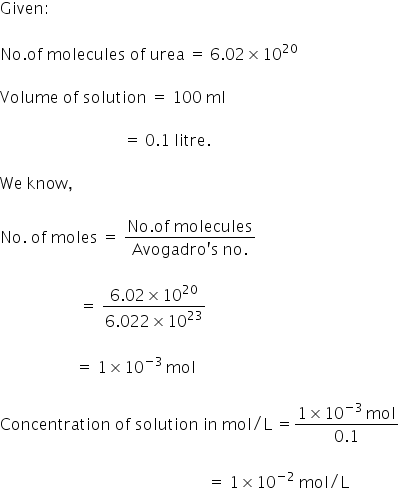
The concentration of urea solution is 1×10-2 mol/L
Answered by Varsha | 22 May, 2018, 09:45: AM
Concept Videos
CBSE 11-science - Chemistry
Asked by manikandanragul1 | 11 Apr, 2024, 09:02: AM
CBSE 11-science - Chemistry
Asked by nikhithaguguloth14 | 29 Mar, 2024, 08:15: PM
CBSE 11-science - Chemistry
Asked by sumedhasingh238 | 27 Mar, 2024, 11:04: PM
CBSE 11-science - Chemistry
Asked by avijotsingh946431 | 22 Feb, 2024, 05:36: PM
CBSE 11-science - Chemistry
Asked by gurmelsinghray | 21 Feb, 2024, 08:43: AM
CBSE 11-science - Chemistry
Asked by bablipanwar893 | 01 Jul, 2023, 12:25: PM
CBSE 11-science - Chemistry
Asked by saijagdale9 | 19 Jun, 2023, 02:34: PM
CBSE 11-science - Chemistry
Asked by kdimple765 | 17 Jul, 2022, 01:24: PM
CBSE 11-science - Chemistry
Asked by alfirozislam900 | 03 Jul, 2022, 01:24: PM
CBSE 11-science - Chemistry
Asked by alfirozislam900 | 03 Jul, 2022, 01:23: PM




