ICSE Class 9 Answered
400 g of wax at 10 deg. celcius is heated to 80 deg. celcius, when it starts melting.On complete melting wax is further heated so that temp. rises to 130 deg. celcius.Calculate (i) heat energy reqd. to bring the wax to its melting point (ii) heat required to melt the wax (iii) heat energy reqd. to bring the molten wax to 130 deg. celcius ( sp. heat capacity of solid wax = 1.5 J/g/c. sp. heat capacity of liquid wax = 1.8 J/g/c and sp. latent heat of wax = 80 J/g)
Asked by nisha_vini29 | 22 Jun, 2016, 10:52: AM
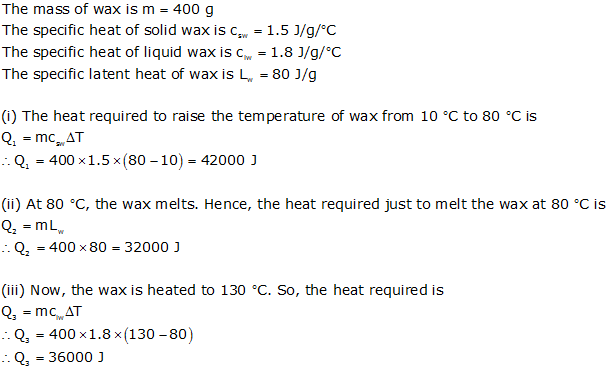
Answered by Romal Bhansali | 23 Jun, 2016, 12:20: PM

