CBSE Class 12-science Answered
3.6M sulphuric acid solution is 29% sulphuric acid {H2SO4} . calculate the DENSITY of the solution
Asked by pardeepkumar2281 | 13 May, 2018, 08:12: AM
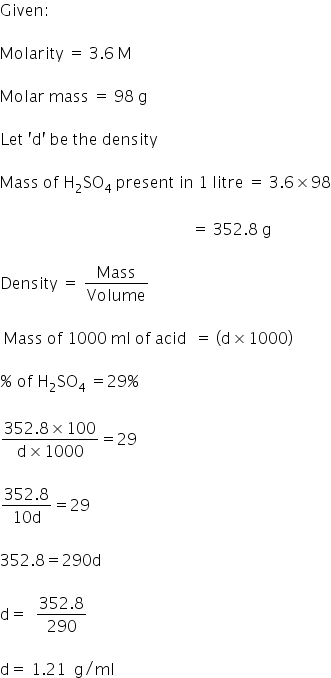
Density of solution is 1.21 g/ml.
Answered by Varsha | 14 May, 2018, 11:17: AM
Concept Videos
CBSE 12-science - Chemistry
Asked by hannamaryphilip | 17 Apr, 2024, 11:20: PM
CBSE 12-science - Chemistry
Asked by sameerteli003 | 08 Apr, 2024, 11:48: PM
CBSE 12-science - Chemistry
Asked by saritanohar22 | 13 Jan, 2024, 01:25: PM
CBSE 12-science - Chemistry
Asked by kamlesh.kumar.malee | 20 Dec, 2023, 06:59: AM
CBSE 12-science - Chemistry
Asked by shamiyaali732 | 26 Sep, 2023, 02:00: AM
CBSE 12-science - Chemistry
Asked by elabarman58 | 23 Jan, 2023, 09:39: AM
CBSE 12-science - Chemistry
Asked by gauravrastogi577 | 16 Aug, 2022, 06:18: PM
CBSE 12-science - Chemistry
Asked by gauravrastogi577 | 16 Aug, 2022, 06:17: PM
CBSE 12-science - Chemistry
Asked by shiv.pama83 | 27 Nov, 2021, 05:50: AM
CBSE 12-science - Chemistry
Asked by mdarsadazizh | 31 Aug, 2021, 01:02: PM







