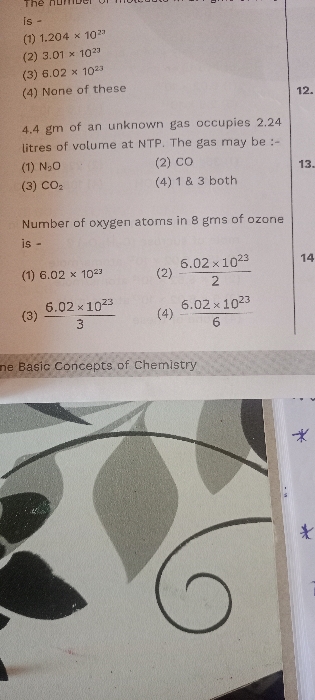NEET Class neet Answered
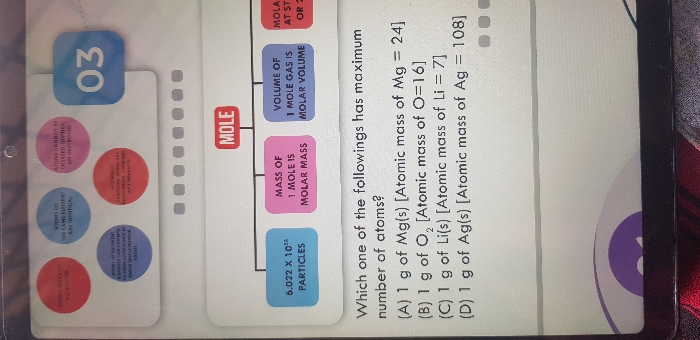
342g of 20% by mass of b a Ba(OH)2 solution (sp. gr.=0.57) is reacted with 1200 mL of 2M HNO3. If the final density of solution is same as pure water then molarity of the iron in resulting solution which decides the nature of the above solution is— (1)0.25M,(2)0.5M,(3)0.888M,(4)none of these.
Asked by patra04011965 | 24 May, 2019, 08:52: AM
The mass of Ba(OH)2 is 342 g.
The molar mass of Ba(OH)2 is 171 gm
Hence,
1 mole of Ba(OH)2 is the molar mass of Ba(OH)2 is 171 gm
So, for 342 g. wil be x
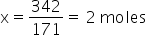
We know 100% by mass is 2moles hence 20% will be x.
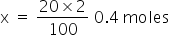
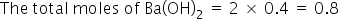
As given the molarity of HNO3 is 2M, So, the total moles=
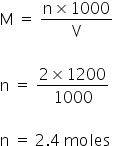
Total acidic excess moles = 2.4 - 0.8 = 1.6
The total volume of Ba(OH)2 is 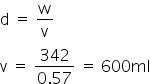
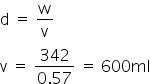
The volume of HNO3 is 1200ml
Hence the total volume of Ba(OH)2 = 1200+600 =1800 ml
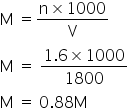
Answered by Ramandeep | 24 May, 2019, 11:02: AM
NEET neet - Chemistry
Asked by biswassayan8464 | 21 Apr, 2024, 11:30: AM
NEET neet - Chemistry
Asked by mahendar160786 | 16 Apr, 2024, 09:23: PM
NEET neet - Chemistry
Asked by muskannawab11 | 14 Apr, 2024, 03:13: PM
NEET neet - Chemistry
Asked by tarasingrathod63 | 07 Apr, 2024, 01:07: PM
NEET neet - Chemistry
Asked by fathimahusna6122 | 05 Apr, 2024, 10:25: AM
NEET neet - Chemistry
Asked by vaka.aruna1979 | 23 Mar, 2024, 04:18: AM
NEET neet - Chemistry
Asked by fathimahusna23042004 | 03 Mar, 2024, 08:56: AM
NEET neet - Chemistry
Asked by drkeshavkhandagle | 18 Jan, 2024, 08:10: PM
NEET neet - Chemistry
Asked by yogitakumawat | 21 Dec, 2023, 10:31: PM