JEE Class main Answered
1.101 ques

Asked by lovemaan5500 | 30 Jan, 2019, 11:59: AM
Given:
Mass of Glauber's salt dissolved = 8.0575 × 10-12 kg
= 80.57 gm
Molecular formula of Glauber's salt = Na2SO4.10H2O
Molecular weight of Glauber's salt = 322 g/mol
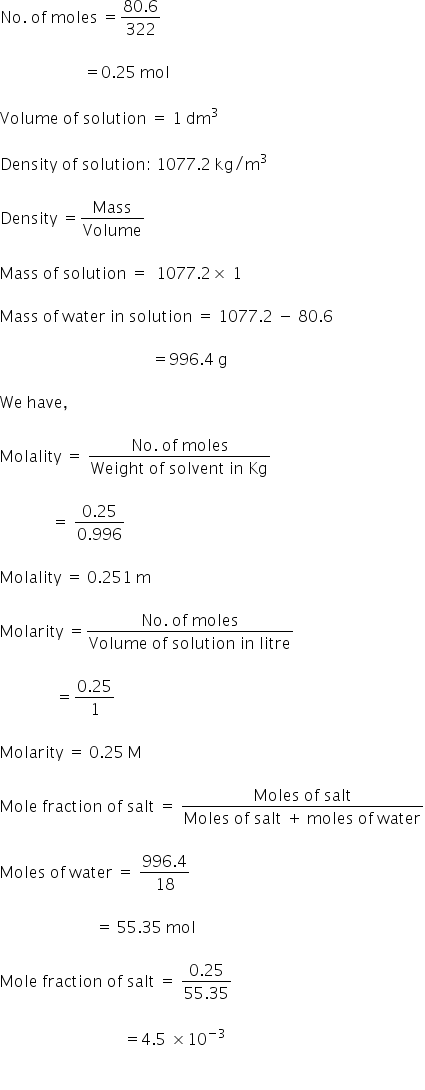
Molality = 0.251 m
Molarity = 0.25 M
Moles fraction = 4.5 × 10-3
Answered by Varsha | 30 Jan, 2019, 04:26: PM
JEE main - Chemistry
Asked by amarnathreddyp19 | 29 Mar, 2024, 06:47: AM
JEE main - Chemistry
Asked by atharvamane801 | 14 Jan, 2024, 12:07: PM
JEE main - Chemistry
Asked by bhyogita884 | 12 Jul, 2022, 02:55: AM
JEE main - Chemistry
Asked by abdulraqeeb437 | 16 Jun, 2022, 08:38: PM
JEE main - Chemistry
Asked by akshatmi2005 | 21 May, 2021, 02:23: PM


