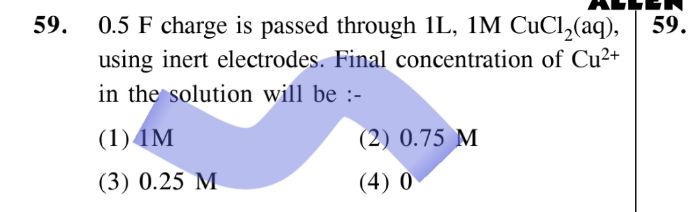
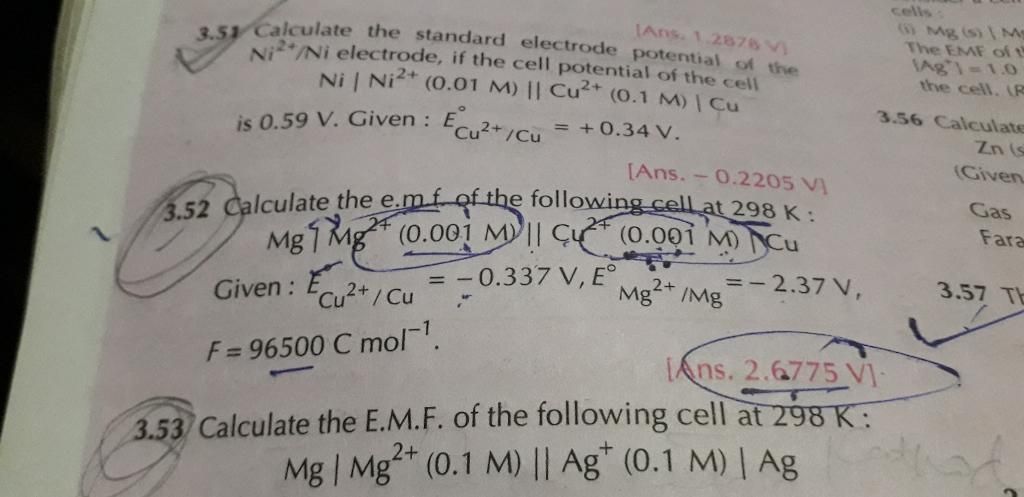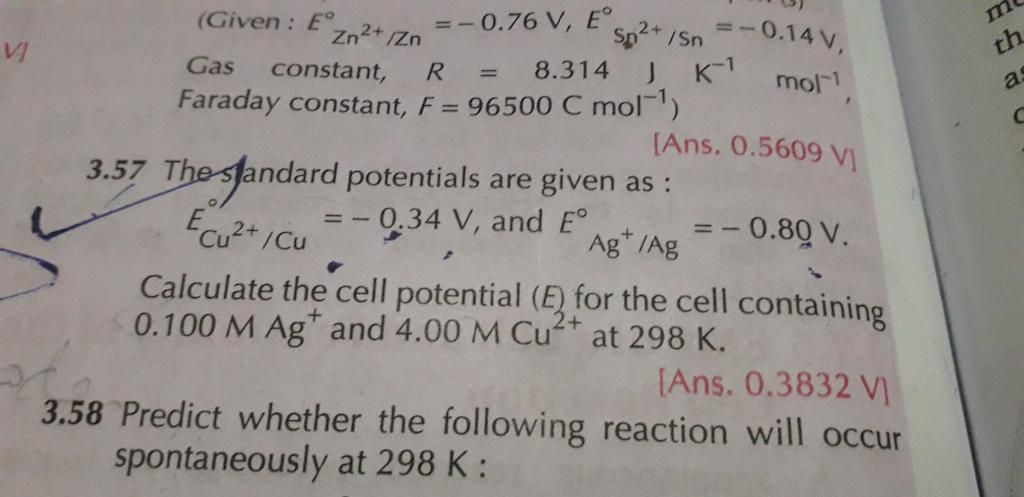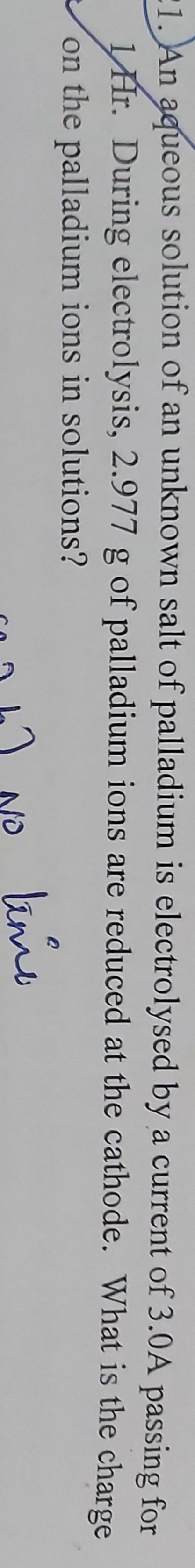CBSE Class 12-science Answered
100 ml of a neutral solution containing 0.2 g of copper was electrolysed till the whole of copper was deposited. The current strength was maintained at 1.2 amperes and the volume of solution was maintained at 100ml. Assuming 100% efficiency, find out the time taken for deposition of copper. [Atomic weight of copper = 63.58]
Asked by Topperlearning User | 22 Jun, 2016, 01:54: PM
W = 0.2; I = 1.2 amperes
Z for Cu = 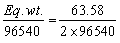
We know that W = Z × I × t
t = 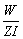
Substituting the values in the above equation, t = 
Answered by | 22 Jun, 2016, 03:54: PM
Concept Videos
CBSE 12-science - Chemistry
Asked by avaneesh5116 | 07 Aug, 2020, 05:24: PM
CBSE 12-science - Chemistry
Asked by harshpareek696 | 01 Aug, 2020, 04:01: PM
CBSE 12-science - Chemistry
Asked by sulaikhasulu393 | 22 Jun, 2020, 08:52: AM
CBSE 12-science - Chemistry
Asked by vasudesetti123 | 23 May, 2020, 08:13: PM
CBSE 12-science - Chemistry
Asked by sakthisivasakthi1978 | 31 Oct, 2019, 09:53: PM
CBSE 12-science - Chemistry
Asked by lovemaan5500 | 19 Aug, 2019, 11:14: PM
CBSE 12-science - Chemistry
Asked by lovemaan5500 | 19 Aug, 2019, 11:13: PM
CBSE 12-science - Chemistry
Asked by lovemaan5500 | 19 Aug, 2019, 11:12: PM
CBSE 12-science - Chemistry
Asked by lovemaan5500 | 19 Aug, 2019, 11:11: PM
CBSE 12-science - Chemistry
Asked by jain.pradeep | 19 Jul, 2019, 09:49: PM