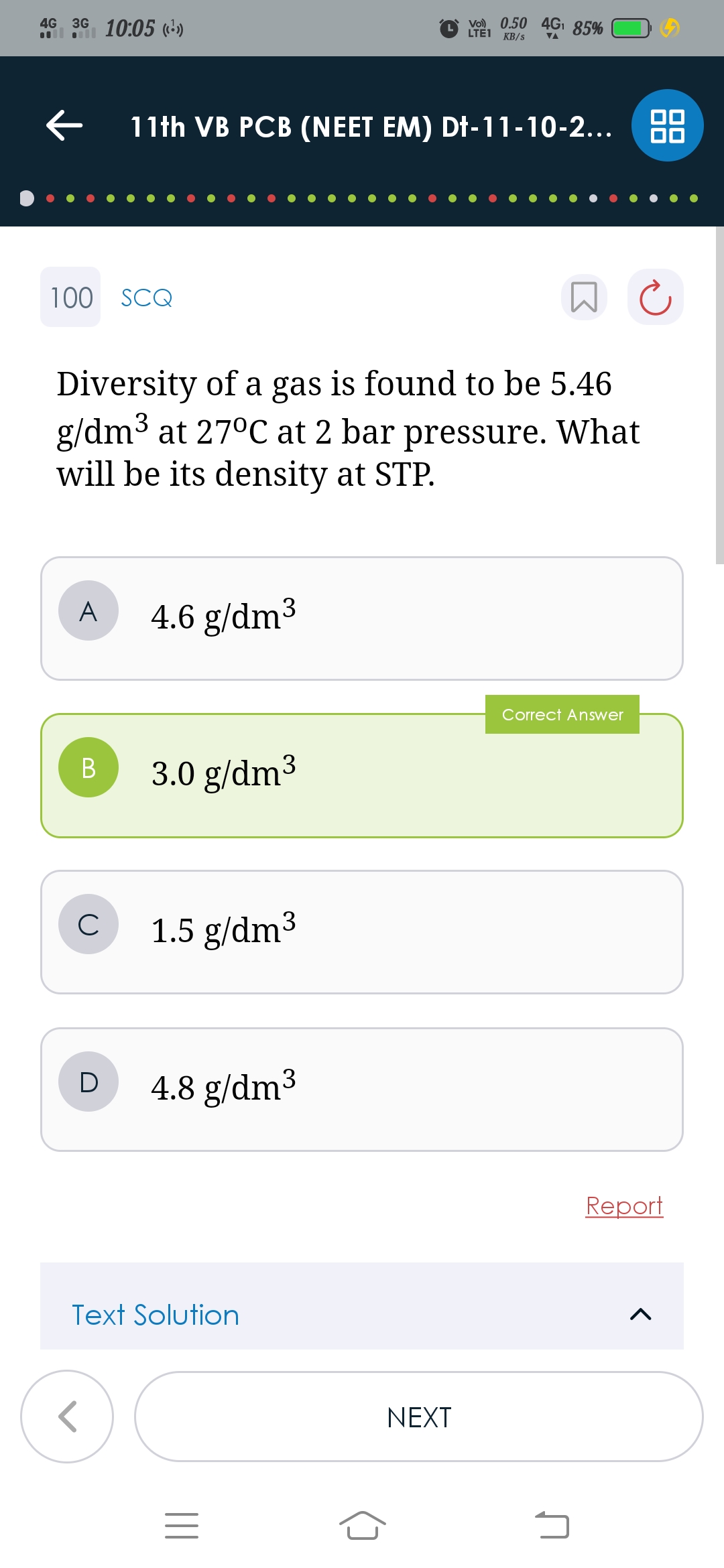
CBSE Class 11-science Answered
Let the volume of CH4 = x ml and volume of C2H4 = y ml
Hence volume of CO2 = (10 - x - y) ml

Since after the explosion, there was contraction of 17ml; this means that when the explosion takes place at that time water is in gaseous state, after the explosion water vapours converted into liquid state and the volume of liquid water is assumed to be zero.
Hence 2x + 2y = 17 .. (a)
Since KOH absorbs CO2 gas when the gaseous mixture is treated with KOH further reduction in volume is 14 ml, hence volume of CO2 in gaseous mixture is 14 ml.
10 - x - y + x + 2y = 14 .. (b)
10 + y = 14
Y = 4 ml
On putting this value in equation (a), we get
x = 4.5
So, volume of CH4 = 4.5 ml
Volume of C2H4 = 4 ml
Volume of CO2 = (10 - 4 - 4.5) = 1.5 ml