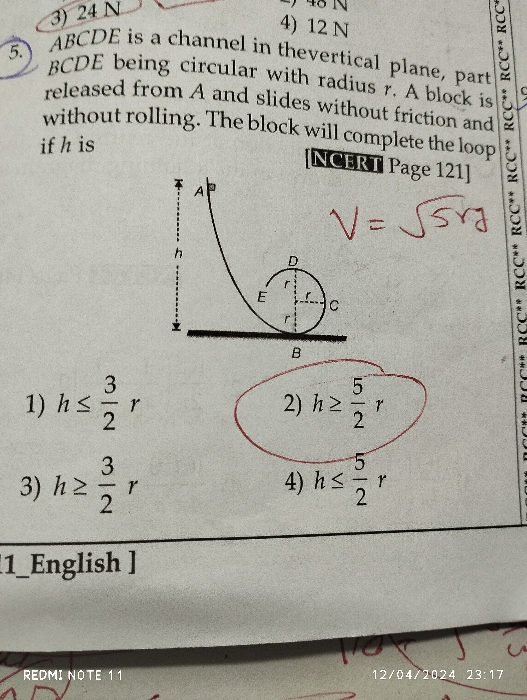NEET Class neet Answered
1 kg of water at 373 K is converted into steam at same temperature. Volume of 1 cm3 of water become 1671 cm3 on boiling. What is the change in the internal energy of the system if the latent heat of vaporisation of water is 5.4×10^5 cal/kg?
Temp and pressure to constant .How to know whether isobaric or isothermal?
Asked by vijaygireesh | 23 Mar, 2020, 12:38: PM
Conversion of water at 373 K to steam at same temperature is isothermal process.
we can not keep pressure also constant, because volume becomes 1671 times while temperature remains constant
Change in internal energy is the enrgy absorbed as latent heat of vapouraisation.
Change in internal energy ΔU for a 1 kg water at 373 K to become steam at same temperature is given by
ΔU = 5.4 × 105 / 4.2 = 1.286 × 105 J
Answered by Thiyagarajan K | 23 Mar, 2020, 01:09: PM
Application Videos
NEET neet - Physics
Asked by praveenpriya000079 | 18 Apr, 2024, 07:24: AM
NEET neet - Physics
Asked by gouranshi84 | 17 Apr, 2024, 05:23: PM
NEET neet - Physics
Asked by sojusvi | 17 Apr, 2024, 01:12: PM