CBSE Class 11-science Answered
1. Enthalpy of combastion of benzen ,carbon,hydrogen,are 3264.5kj,-393.5kj, -285.8kj respectively? calculate the enthalpy of formation of benzen.
Asked by rahmani | 27 Jan, 2011, 12:00: AM
Dear Student
The reaction for the formation of benzene is
6C(s) + 3H2(g) -------> C6H6
In general we can understand the reaction path as follows:
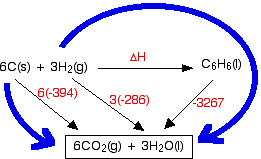
1. C6H6 + O2(g) -----> CO2(g) + H2O [delta]H = - 3264.5 kJ/mol
2. C(s) + O2(g) -----> CO2(g) [delta]H = - 393.5 kJ/mol
3. H2(g) + 1/2 O2(g) -----> H2O(l) [delta]H = - 285.8 kJ/mol
3. H2(g) + 1/2 O2(g) -----> H2O(l) [delta]H = - 285.8 kJ/mol
According to the Hess's Law:
ΔH - 3264.5 = 6(-393.5) + 3(-285.8)
Rearranging and solving:
ΔH = 3264.5 + 6(-393.5) + 3(-285.8)
= 3264.5- (2361 +857.4)
ΔH = +46.1 kJ mol-1
We hope that clarifies your query.
Regards
Team
Topperlearning
Answered by | 28 Jan, 2011, 10:59: AM
Concept Videos
CBSE 11-science - Chemistry
Asked by shreyasharma94.11dga | 23 Mar, 2022, 11:42: PM
CBSE 11-science - Chemistry
Asked by adipadmakarri | 05 Dec, 2021, 11:38: AM
CBSE 11-science - Chemistry
Asked by mrassam2711 | 15 Jan, 2021, 11:54: PM
CBSE 11-science - Chemistry
Asked by rajpalsingh5892 | 04 Jun, 2020, 08:29: PM
CBSE 11-science - Chemistry
Asked by navkirank023 | 02 Jun, 2020, 07:39: PM
CBSE 11-science - Chemistry
Asked by sangmeshvk2909 | 07 May, 2020, 07:07: AM
CBSE 11-science - Chemistry
Asked by supreetjoshi28 | 04 May, 2020, 11:12: AM
CBSE 11-science - Chemistry
Asked by bvk6200718 | 18 Apr, 2020, 07:55: PM
CBSE 11-science - Chemistry
Asked by binalmarakana16 | 10 Apr, 2020, 10:00: PM
CBSE 11-science - Chemistry
Asked by binalmarakana16 | 10 Apr, 2020, 02:41: PM







