CBSE Class 11-science Answered
1) a sample of hydrogen gas as volume 906 cm cube at 27 degree celsius. calculate the temperature at which it will occupy 500 cm cube of volume?
2) the density of the gas was found to be 3.43 gram per litre at 300 degree Kelvin and one atmosphere. calculate the molar mass when R = 0.0821 litre atmosphere per Kelvin per mole?
3) a 5 litre flask contain hundred gram of Sulphur trioxide and one gram of helium at 20 degree Celsius. calculate the partial pressure of sulphur trioxide and helium and also total pressure?
4) how many molecules of ideal gas are there in 1 into 10 power minus 3 DM cube at STP?
5) calculate the total pressure in 10 litre cylinder which contain 0.4 gram of helium,1.6 gram of Oxygen and 1.4 gram of Nitrogen at 27 degree Celsius also calculate partial pressure of the helium gas in the cylinder. assume all the gas behave ideal?
Asked by hemanttkumarr | 12 Nov, 2017, 02:25: PM
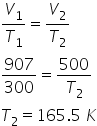
Answered by Varsha | 12 Nov, 2017, 11:16: PM
Concept Videos
CBSE 11-science - Chemistry
Asked by Prashant Malik | 02 Apr, 2018, 11:30: AM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 21 Apr, 2015, 01:21: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 17 Jun, 2016, 12:05: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 21 Apr, 2015, 01:27: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 21 Apr, 2015, 01:28: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 21 Apr, 2015, 01:30: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 21 Apr, 2015, 01:31: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 17 Jun, 2016, 12:05: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 21 Apr, 2015, 01:36: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 17 Jun, 2016, 12:05: PM




