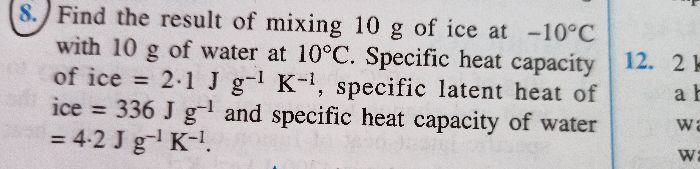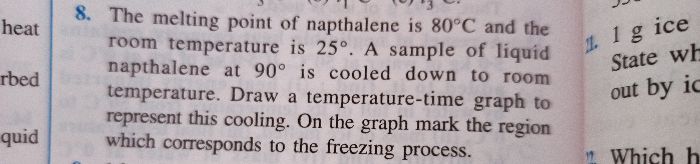ICSE Class 10 Answered
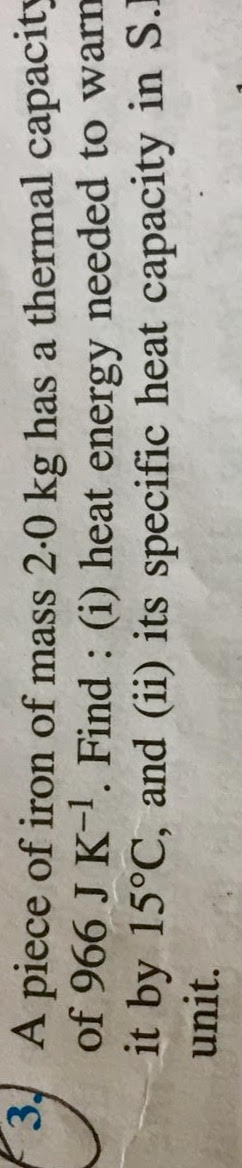
Pls advise solution
Asked by vanij1301 | 15 Feb, 2017, 02:28: PM
(i) Thermal capacity = 966 J° C-1 = 966 J K-1
Rise in temperature = 15° C = 15 K
Heat required = Thermal capacity × Rise in temperature
= 966 J K-1 × 15 K
Heat required = 14490 J
(ii) Specific heat capacity of iron = Thermal capacity / Mass of iron
= 966 J K-1 / 2 kg
Specific heat capacity of iron = 483 Jkg-1 K-1
Answered by Yashvanti Jain | 15 Feb, 2017, 06:40: PM
Concept Videos
ICSE 10 - Physics
Asked by imunilu786 | 29 Oct, 2023, 02:35: PM
ICSE 10 - Physics
Asked by surendrakumarclw | 20 Mar, 2021, 06:16: PM
ICSE 10 - Physics
Asked by anuradhastudent12 | 27 Feb, 2021, 08:33: PM
ICSE 10 - Physics
Asked by chitrachongdar07 | 08 Dec, 2020, 10:55: AM
ICSE 10 - Physics
Asked by charan3802 | 23 Oct, 2020, 10:29: AM
ICSE 10 - Physics
Asked by khushalkumarsk | 17 Aug, 2020, 12:37: PM
ICSE 10 - Physics
Asked by nilesh.dhote74 | 13 Jul, 2020, 12:38: PM
ICSE 10 - Physics
Asked by abhinabasarkar.abhinaba | 17 Jun, 2020, 09:24: PM
ICSE 10 - Physics
Asked by nilesh.dhote74 | 10 Jun, 2020, 03:00: PM
ICSE 10 - Physics
Asked by chaitalisen977 | 19 May, 2020, 03:32: PM