CBSE Class 12-science Physics Alpha Particle Scattering
- What was the aim of Rutherford's alpha particles scattering experiments? Who performed the experiment? What was the main outcome of the experiments?
-
Explain how Rutherford’s exp on scattering of
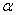 particles led to the estimation of the size of the nucleus.
particles led to the estimation of the size of the nucleus.
- What are the drawbacks of Rutherford's atom model?
- As per Rutherford's atom model, what is the total energy of an electron revolving in a circular orbit of radius r?
- What is the main feature of Rutherford's atom model?
-
What is the main conclusion of
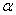 -particles scattering experiments?
-particles scattering experiments?
- Define distance of closest approach?
- What would change in the gold foil experiment if it was beta not alpha particles ? Would the negatively charged particles be attracted to the positive nucleus?
- In Rutherford's experiment, a thin gold foil was bombarded with alpha particles. According to Thomson's "plum-pudding" model of the atom, what should have happened?
- How is the impact parameter b defined in scattering?

