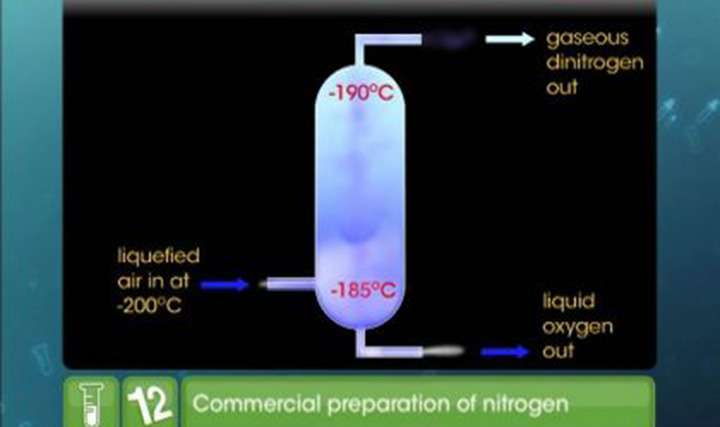
CBSE Class 12-science Chemistry Nitrogen and Its Compounds
Dive deeper into what is nitrogen and understand why it is an important topic in Chemistry with our online study materials. TopperLearning’s concept videos on CBSE Class 12 Chemistry The p-Block Elements – Nitrogen and its Compounds will help you immensely with conceptual clarity. Through our video lessons and topic notes, you can learn the uses and properties of ammonia, dinitrogen and nitric acid.
You can practise nitrogen-related questions and answers using our textbook solutions such as NCERT solutions. Exam preparation is critical for CBSE Class 12 Science students, and therefore, we have past years’ papers and sample papers with solutions to boost your exam confidence.
- which halide do not form covalent bond give reasons
-
H3pO4 ka total charge

-
pls explain
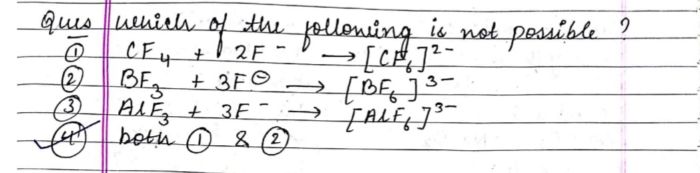
- why nitrogen have long range of oxidation state?
- We know that nitrogen can't have +5 oxidation state,so why N2O5 exists?
- Why NO does not dimerise even it has odd electron?
- Explain the structures of BCl3 and PH3 with the help of theory of hybridisation.
- A gas X is formed by heating ammonium nitrite. X can also be obtained by heating barium azide. Mg metal burns in X to form Y. Y reacts with water to form a gas zZwhich turns red litmus blue. Identify X,Y and Z. Write all the reactions involved.
- A gas is obtained by the reaction of magnesium nitride with water. It acts as a lewis base and turns red litmus blue. It reacts with CuO as a reducing agent. It is obtained from two elements by reacting at 700K and high pressure. It is highly soluble in water. It forms blue coloured solution with copper sulphate. Identify the gas and write all the reactions involved.
-
GROUP 15 ELEMENT QUES .
1) An unknown salt X react with hot conc.
to produce a brown coloured gass which intensifies on addition on copper turnings . on adding dilute ferrous sulphate olution to an aqueous solution to an aquaeous solution of X and then carefully adding conc.
along the sides of testtube , a brown complex Y i formed at the interface between the solution and
. identify X and Y and write the reactions involved . 2) A transuclent waxy solid A on heating it on inert atmosphere is converted into its allotropic form B . Allotrope A on reaction with very dilute aqueous NaOH LIBERATES a highly poisonous gas C having rotten fih mell . The ubstance A reacts with excess of chlorine to form D which hydrolysis to form compound E .Identify A to E . Also write the reaction involved Thankyou