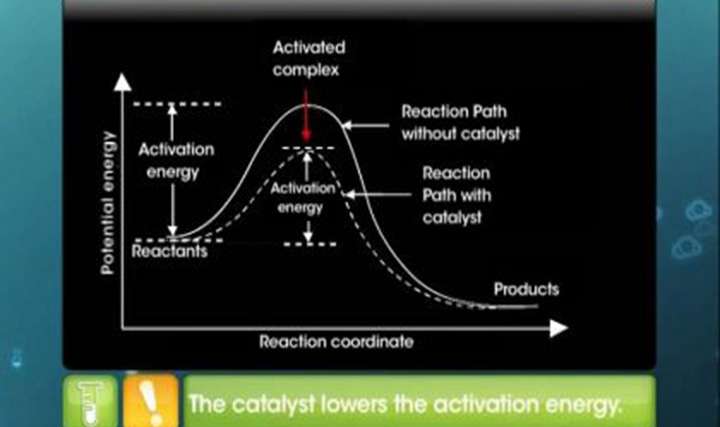
CBSE Class 12-science Chemistry Catalysis
Enjoy learning CBSE Class 12 Science Chemistry Surface Chemistry – Catalysis at TopperLearning. Chemistry won’t be mind boggling if you have the right learning materials to learn and revise concepts. Learn to associate concepts such as catalyst, activation energy etc. with visuals present in our video lessons. It will help you to remember the key concepts in Surface Chemistry during your exam.
At TopperLearning, you’ll get revision notes, practice tests and CBSE Class 12 Science Chemistry textbook solutions to study the topic of catalysis easily. Write and practise the solutions for important questions using our expert solutions for Chemistry like previous years’ papers and sample papers. For additional help, go through the doubts and solutions in Chemistry posted in the UnDoubt section of our website.
-
in homogeneous catalysis both catalyst and reactants have same?

- what is catalysis
- examples of hetrogenous catalyst
- Example of heterogeneous catalysis
- what is catalyst? Give an example of reaction to show.
- name the two groups into which phenomenon of catalysts can be divided give an example of each group with chemical equation involved
- Explain with the help of example INDUCED CATALYST
- Explain the THEORY OF HETEROGENOUS CATALYSIS in detail with the help of examples
- What will happen if DISORPTION OF PRODUCT do not takes place in case of HETEROGENOUS CATALYSIS ? Explain in detail.
- Give characteristic of a good catalyst.