CBSE Class 12-science Chemistry Aldehydes and Ketones
Revise CBSE Class 12 Science Chemistry Aldehydes, Ketones and Carboxylic Acids – Aldehydes and Ketones with our concept videos in which our Chemistry experts make important chemical reactions easier with appropriate visual elements. You will also come across valuable exam preparation strategies in our video lectures. In addition, learn the methods of preparation of ketones and aldehydes with our topic notes.
Study about aldehydes and ketones for your board exam with sample papers, practice tests, textbook solutions and more. Enjoy flexible Chemistry revision sessions with TopperLearning’s CBSE Class 12 Science Chemistry online learning materials which are available 24/7.
- C-C=C-CHO ka iupac name
- identify the product when ch3ch=ch-ch2-ch2-c=o-ch3 reacts with following reagents ( a) lialh4/d2o (b) nabh4 (c) c2h5mgbr
- explain the following named reaction aldol condensation
- sir/madam, 1. in NCERT chemistry books in organic chemistry sometimes hydrolysis rxn takes places by acid and water(H2SO4 + H2O/ H3O+/H+ + H2)), base with water(NaOH + H2O/ KOH + H2O) and other times only with water(H2O) what is the difference between the three and when do we use them in rxns ? hydrolysis means breaking or cleavage of compounds using water.... am I right? 2. Is Aldol rxn and condensation only limited to 2 molecules of same aldehyde compound and 2 molecules of same ketone compound? then does it mean that it undergoes self condensation? And cross Aldol rxn and condensation is carried out by two different molecules of aldehydes and two different molecules of ketones. is cross Aldol rxn and condensation also carried out between one aldehyde and one ketone molecules? request u to plz answer all questions
-
sir/madam,
is KMnO4-KOH the chemical formula for alkaline potassium permanganate?
like in the NCERT if alkylbenzene is reacted with chromic acid (H2CrO4), will there be any intermediate product just like alkylbenzene when reacted with KMnO4-KOH gives potassium salt of carboxylic acid which then further oxidises to carboxylic acid?
also is my formula correct for chromic acid and alkaline potassium permanganate?
pls requested to respond to all my questions
-
IUPAC name

-
Conversions from a-g

- how do we convert esters to aldehyd
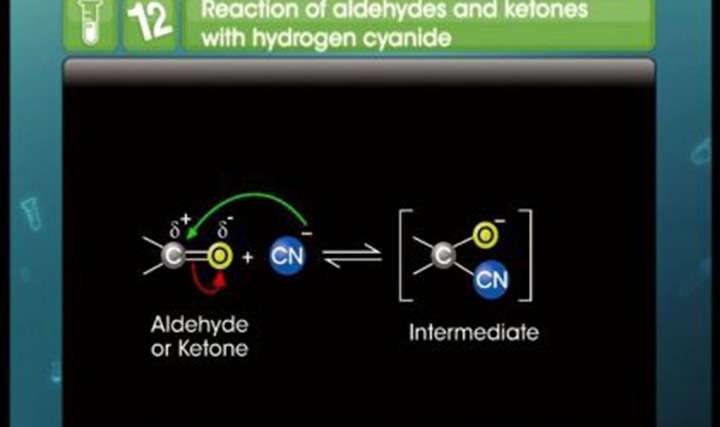
- how is the cabonyl group of aldehyde/ketone affected in the presence of electron donating group like metyl in cyanohydrin reaction?
- an organic compound a with molecular formula c6h6o react with zinc dust to give a hydrocarbon B which upon reaction with CH3Cl in the presence of anhydrous AlCl3 gives C.compound C on oxidation with alkaline KMnO4 gives compound D Compound A on further reaction with NH3 in the presence of anhydrous ZnCl2 gives E identify compound A B C D E and also justify your answer