CBSE Class 11-science - Resonance and Hydrogen Bond Videos
Chemical Bonding and Molecular Structure
This video explains the concept of resonance.
- Explain why sucrose is quite soluble in water though it is a covalent compound.
- Which has higher boiling point o-nitro phenol or p-nitro phenol? Give reason for your answer.
-
The resonating structures of a molecule are given below. Draw the probable hybrid structure.
- SO2 and CO2 are triatomic molecules. Compare their dipole moment. Justify your answer.
- Explain why H2O is liquid while H2S is a gas.
- Give two resonating structure of ozone which satisfy octet rule. Also give probable hybrid structure.
- "BeH2 molecule has zero dipole moment although the Be-H bonds are polar." Explain this sentence on the basis of the concept of dipole moment.
-
i)Out of the following resonating forms of formaldehyde which one is least significant and why?
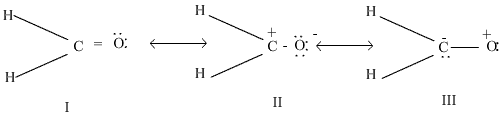 ii) What are the factors on which the dipole moment of polyatomic molecules depends?
ii) What are the factors on which the dipole moment of polyatomic molecules depends?
- The dipole moment of a diatomic molecule is 4.1625 x 10-30cm, express it in Debye units.
- (i) Describe the conditions necessary for hydrogen bonding. (ii) Differentiate between intermolecular and intramolecular hydrogen bonding. (iii) How does energy of the conical structures contribute to the stability of resonance hybrid?

