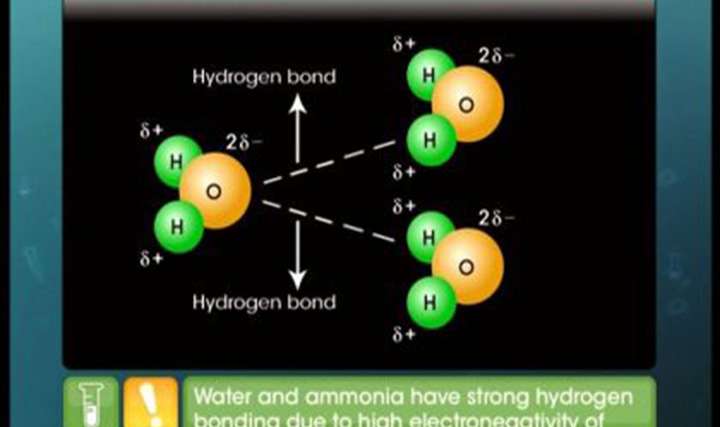
CBSE Class 11-science Chemistry Intermolecular Forces
- why when temperature is increased volume is also increased
- can you give me important question on the topic : states of matter
- Gases exert pressure on the walls of the container why? Explain in one two answer
- Proof: P = hpg
- a gas exert pressure on the walls of container, give reason
- nitrogen filled in 1 kg cylinder @150 bar . then strored it at 0 degree cetrigrade. for 24 hrs. what will be changes in properties of nitrogen.
- On the basis of kinetic theory explain why, ammonium chloride sublimes and goes from solid state directly into vapour state
- What is important in the chapter of states of matter ?
- solubility of gas in liquid is decrease as increase in temperature
- Which substances among the following experiences dipole-dipole intermolecular forces? SiF4, CHCl3, CO2, SO2