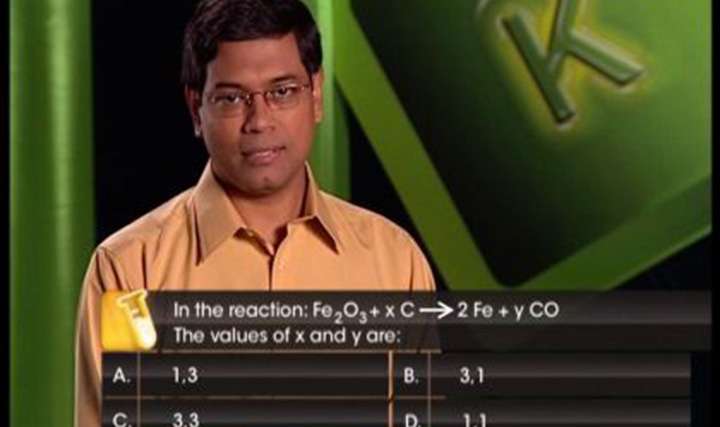CBSE Class 11-science Chemistry Balancing Redox Reactions
- what is the most essential conditions that must be satisfied in a redox reaction
- H2O2+Fe2+=H2O +Fe3+ , give ion exchange method
-
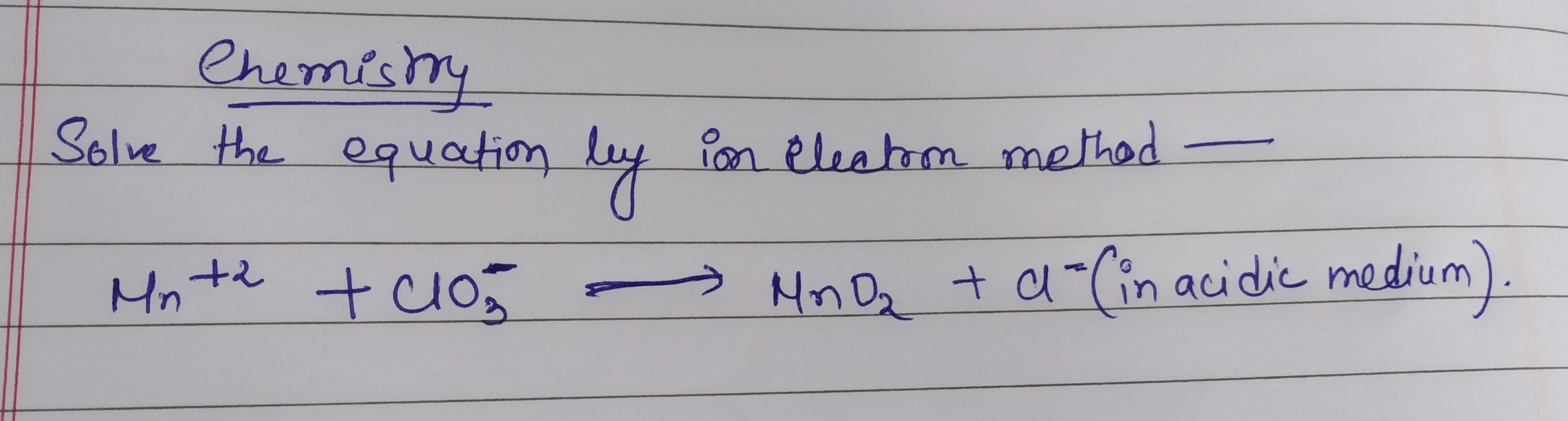
Redox Reaction: solve the following equation by ion electron method in acidic medium
- NO3 (-ve)+I (-ve)+H (+) =NO +I2 +H2O
- magnesium reacts with nitric acid to give magnesium nitarate and nitrous oxide gas and liquid water balance this by oxidation number method
- H2S + KMnO4 + H2SO4 → S+ MnSO4 + KHSO4 + H2O Balance by oxidation number method step by step explain please
- Zn + HNO3 = Zn(NO3) + NO2 + H2O balance this equation by oxidation number method step wise
- MO4 minus + I gives MnO2+I2
-
Solve it

- Define oxidation in terms of oxidation numbers.