CBSE Class 12-science Answered
1. Compound 'A' gives a positive test with Tollen's reagent. This means it is an aldehyde.
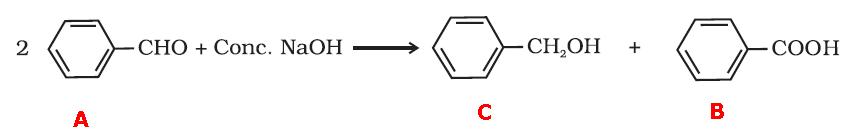
2. The compound 'A' reacts with concentrated solution of NaOH to give compounds 'B' and 'C'. This is cannizaro's reaction. It means that the aldehyde does not have an α - hydrogen.
3. The formula of the compound A is C7H6O. The ratio of the number of carbons to hydrogen indicates that it has a benzene ring.
Now, start back tracking the structures.
4. D is benzene. It is formed by the reaction of carboxylic acid B with sodalime. This is decarboxylation reaction.
The compound 'B'(C7H6O2) is a carboxylic acid while the compound 'C'(C7H8O) is an alcohol.
Compound 'B' can also be prepared by oxidation of compound 'A'. This means the hydrocarbon part is same as that of carboxylic acid and alcohol.
Compound 'A' reacts with concentrated solution of NaOH to give compounds 'B' and 'C'.
The compound 'B'(C7H6O2) is a carboxylic acid while the compound 'C'(C7H8O) is an alcohol.
The reaction is