CBSE Class 12-science Answered
Write the chemistry of recharging the lead storage battery
Asked by vinodjoshi112233 | 09 Jun, 2017, 03:46: PM
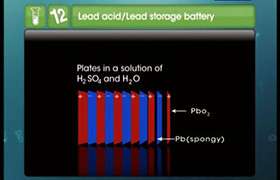
In lead storage battery, when H2SO4 is used up during the discharge, the density of H2SO4 falls. When it falls below 1.20 cm-3, the battery needs recharging.
Duriing recharging, the cell is operated like an electrolytic cell, i.e., now electrical energy is suppiled to it from a external source. The electrode are the reverse of those that occur during discharge:
At cathode: PbSO4(s) + 2e- → Pb(s) + SO42-(aq) (Reduction)
At anode: PbSO4(s) + 2H2O → PbO2(s) + SO42-(aq) + 4H+(aq) + 2e- (Oxidation)
__________________________________________________________________________
Overall reaction: 2PbSO4(s) + 2H2O → Pb(s) + PbO2(s) + 4H+(aq) + 2SO42-(aq)
Answered by Prachi Sawant | 09 Jun, 2017, 05:06: PM
Concept Videos
CBSE 12-science - Chemistry
Asked by Topperlearning User | 16 Apr, 2014, 10:35: AM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 22 Jun, 2016, 02:58: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 22 Jun, 2016, 02:53: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 22 Jun, 2016, 02:53: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM