CBSE Class 11-science Answered
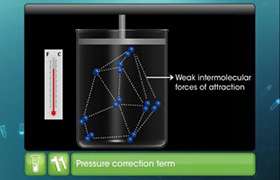
The van der Waals equation contains a pair of constants (a and b) that change from gas to gas. The value of ‘a’ accounts for the intermolecular attractive forces between gas molecules. The magnitude of ‘a’ is indicative of the strength of the intermolecular attractive force. Molecules experiencing the weakest attractive forces will have the smallest ‘a’ constant while those with the strongest attractive forces will have the largest values.
Eg: Consider N2 gas and NH3 gas. N2 gas has non-polar bonds between their components atoms which results in weaker attractions between molecules. In case of NH3 gas hydrogen bonds between molecules which are stronger than non-polar bonds. So NH3 gas has higher ‘a’ constant value (4.170) than N2 gas (1.390).
The factor ‘b’ accounts for the volume occupied by the gas molecules. Small molecular volume results in small b values and a large molecular volume corresponds to a large 'b' constant.
Eg: CH4 gas has higher molecular volume as compare to H2 gas so CH4 gas will show higher ‘b’ constant value (0.04278) while H2 gas will show lower ‘b’ constant value (0.02661).