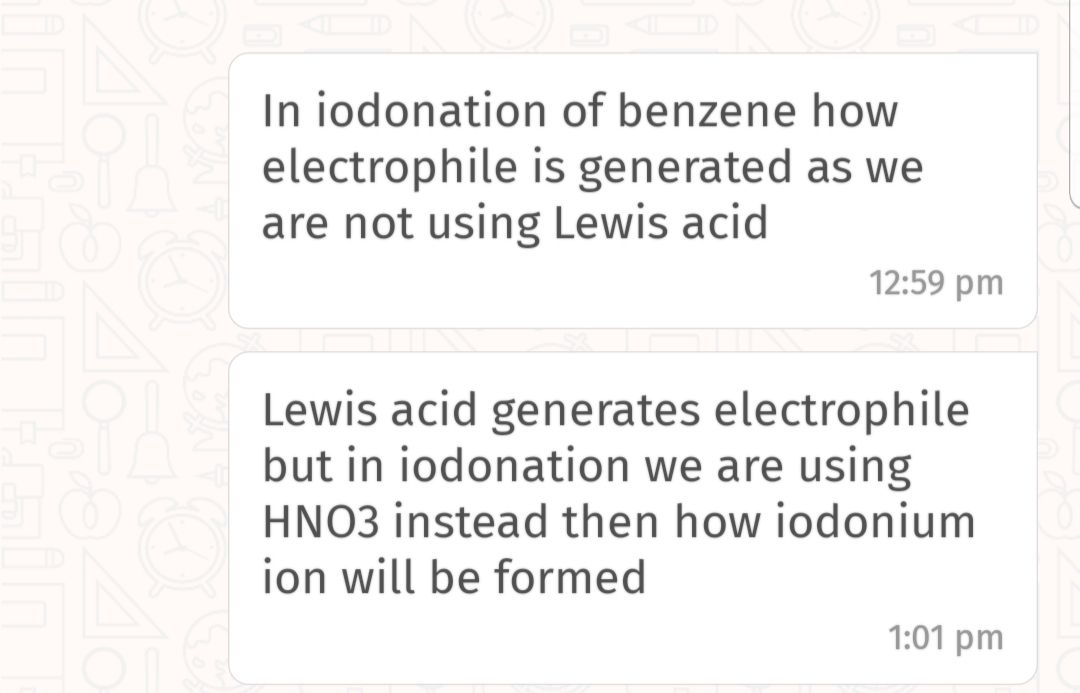
CBSE Class 12-science Answered
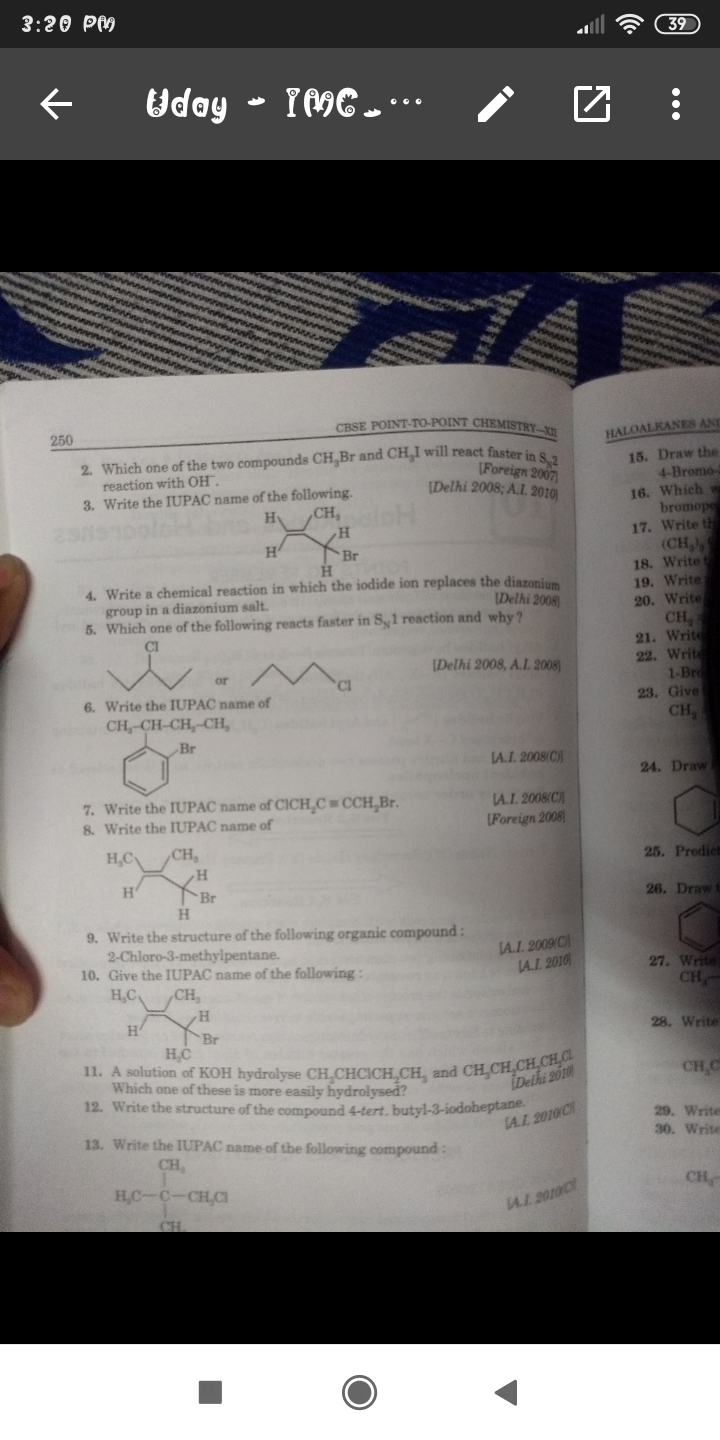
why ZnCl2 is not needed for the tertiary alkane in the reaction:
ROH + HCl = RCl + H2O
Asked by Rahul Mishra | 06 Aug, 2014, 09:48: PM
1.Primary and Secondary alcohols reacts very slowly and at moderate rate respectivly with HCl. While tertiary alcohols undergo the reaction much more faster (Just shaking with Conc.HCl is required).
2.Zinc chloride (ZnCl2) act as a catalyst for this reaction. Lewis acid such as ZnCl2 promote reactions of HCl with primary and secondary alcohols.
3.The ZnCl2 will bind to the oxygen of the hydroxyl group with more efficiency and form the good leaving group.
Answered by Arvind Diwale | 07 Aug, 2014, 01:12: PM
Concept Videos
CBSE 12-science - Chemistry
Asked by roshanisharma200611 | 07 Feb, 2024, 01:18: PM
CBSE 12-science - Chemistry
Asked by surekhas66675 | 02 Sep, 2021, 05:17: PM
CBSE 12-science - Chemistry
Asked by nazimb0313 | 02 Sep, 2020, 09:34: AM
CBSE 12-science - Chemistry
Asked by mastertask199 | 13 May, 2020, 04:08: PM
CBSE 12-science - Chemistry
Asked by jain.pradeep | 03 Feb, 2020, 10:42: PM
CBSE 12-science - Chemistry
Asked by ajaysankhala051 | 11 Sep, 2019, 01:19: PM
CBSE 12-science - Chemistry
Asked by pragyachandraul03 | 23 Aug, 2019, 02:12: PM
CBSE 12-science - Chemistry
Asked by priadkonkar | 21 Jan, 2019, 08:52: PM
CBSE 12-science - Chemistry
Asked by rakeshraghav33 | 21 Jan, 2019, 02:49: PM
CBSE 12-science - Chemistry
Asked by Atulcaald | 16 May, 2018, 02:34: PM




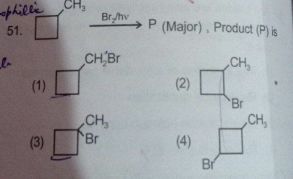
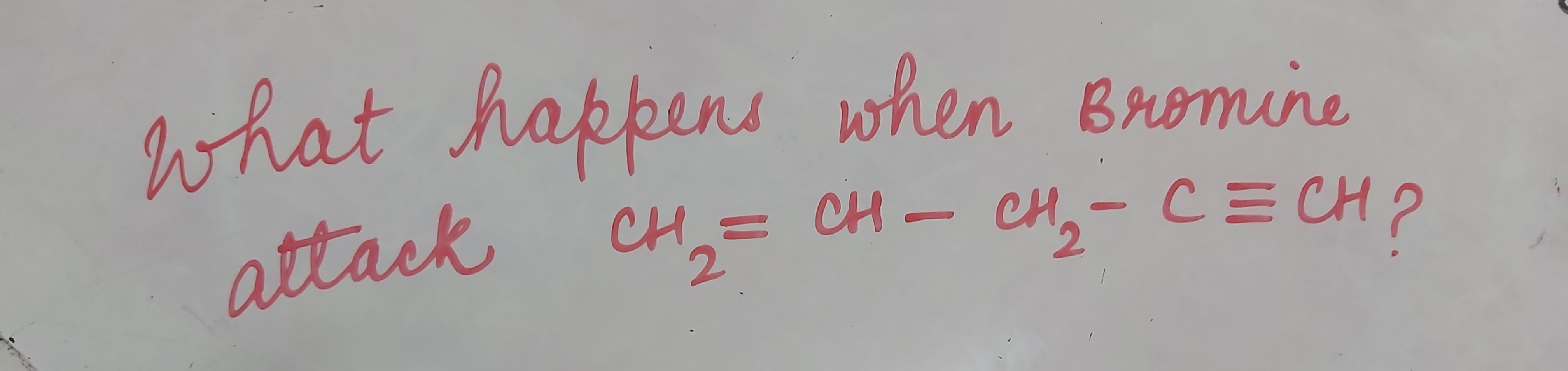
 Total No. of Mono Brominated product :-
Total No. of Mono Brominated product :-