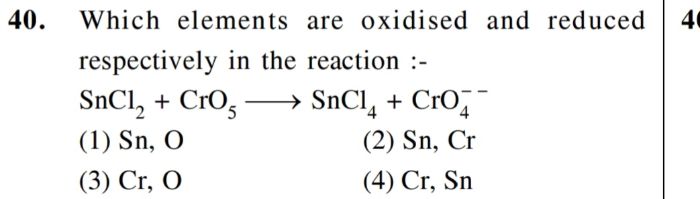CBSE Class 12-science Answered
Why zeff equivalent to screening effect for Fe Co and ni
Asked by Debdulal | 29 Aug, 2019, 04:46: PM
Electronic configuration of first period of d block elements (from Sc to Zn)-
[Ar]3d1-10 4s2
Generally We study Screening effect for valence electrons. when electrons increases in 3d orbitals, these electrons provides more repulsion force to the outermost electrons in 4s. From Sc to Mn, Due to less Number of electrons Repulsion force is not much high so Zeff>screening effect.
In Fe, Co ,Ni number of electrons in 3d orbitals increases and repulsion force becomes almost equal to attraction of nucleus so screening effect is almost equal to Zeff.
In Cu, Zn number of electrons i in 3d orbitals ncreased and It's screening effect becomes higher than Zeff. So in this case Zeff<Screening effect.
Answered by Ravi | 30 Aug, 2019, 03:15: PM
Concept Videos
CBSE 12-science - Chemistry
Asked by anubhutiupadhaya | 27 Feb, 2024, 04:28: PM
CBSE 12-science - Chemistry
Asked by basib61203 | 08 Feb, 2024, 06:03: PM
CBSE 12-science - Chemistry
Asked by ABHILASHA | 04 Mar, 2021, 02:26: AM
CBSE 12-science - Chemistry
Asked by ghoshmahadev037 | 20 Sep, 2020, 11:45: AM
CBSE 12-science - Chemistry
Asked by mufeedatvp2000 | 20 Apr, 2020, 02:53: PM
CBSE 12-science - Chemistry
Asked by patra04011965 | 25 Sep, 2019, 10:22: PM
CBSE 12-science - Chemistry
Asked by patra04011965 | 22 Sep, 2019, 01:42: PM
CBSE 12-science - Chemistry
Asked by jain.pradeep | 30 Aug, 2019, 08:09: AM
CBSE 12-science - Chemistry
Asked by Debdulal | 29 Aug, 2019, 04:46: PM
CBSE 12-science - Chemistry
Asked by Debdulal | 29 Aug, 2019, 04:44: PM