CBSE Class 12-science Answered
Why toluene is more reactive than nitro benzene?
Asked by afiaorpi01 | 22 Mar, 2019, 01:19: AM
In nitrobenzene deactivating group -NO2 is present. It reduces the electron density of benzene group making it less prone to attack by an electrophile.
On the other hand, in toluene ring activating group -CH3 is present which activates ring resulting in increased reactivity for electrophilic addition.
Thus nitrobenzene reacts less readily with electrophilic reagents as compared to toluene.
Answered by Ramandeep | 22 Mar, 2019, 05:07: PM
Concept Videos
CBSE 12-science - Chemistry
Asked by kaziryan.05 | 06 Jul, 2021, 11:31: PM
CBSE 12-science - Chemistry
Asked by mufeedatvp2000 | 15 Apr, 2020, 01:35: PM
CBSE 12-science - Chemistry
Asked by Sudamkalgunde624 | 31 Dec, 2019, 11:38: AM
CBSE 12-science - Chemistry
Asked by prakriti12oct | 19 Nov, 2019, 12:39: PM
CBSE 12-science - Chemistry
Asked by dineshchem108 | 19 Jun, 2019, 09:19: PM
CBSE 12-science - Chemistry
Asked by afiaorpi01 | 22 Mar, 2019, 01:19: AM
CBSE 12-science - Chemistry
Asked by abhitailor158 | 07 Mar, 2019, 04:44: PM
CBSE 12-science - Chemistry
Asked by Atulcaald | 16 May, 2018, 02:44: PM


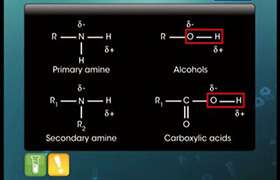

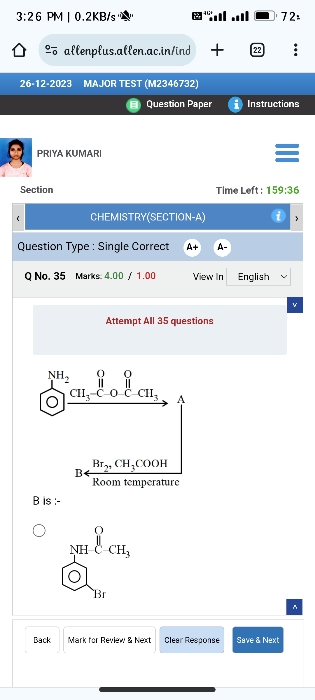
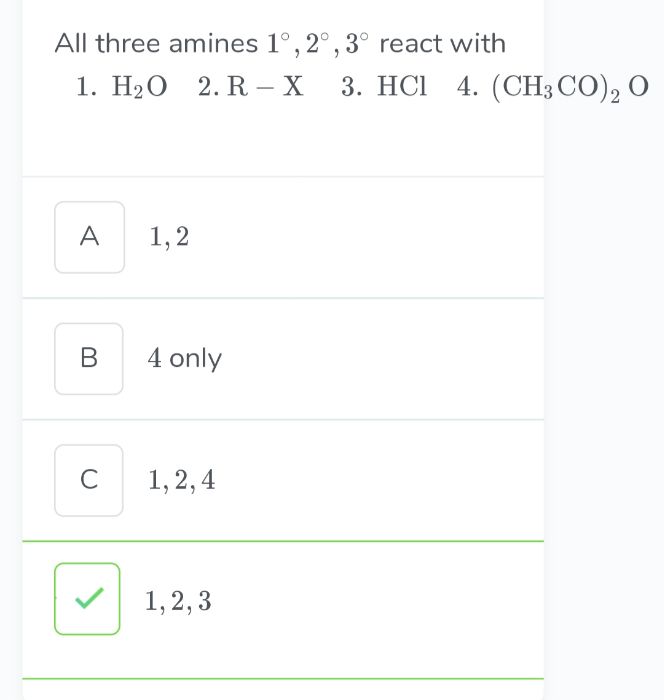
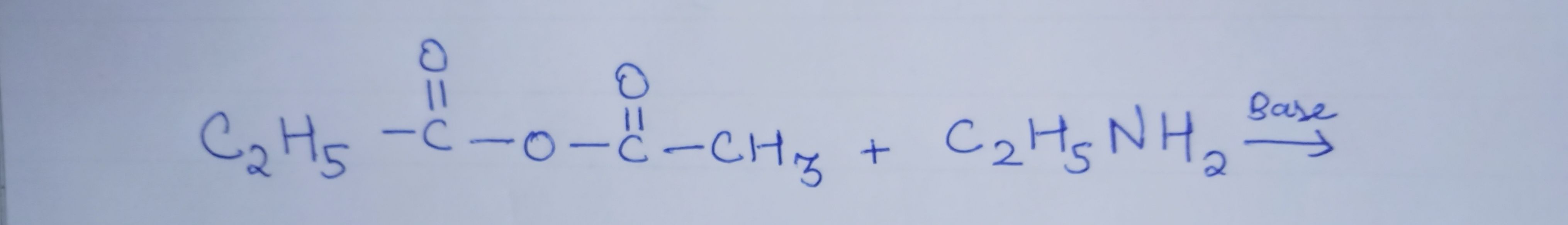
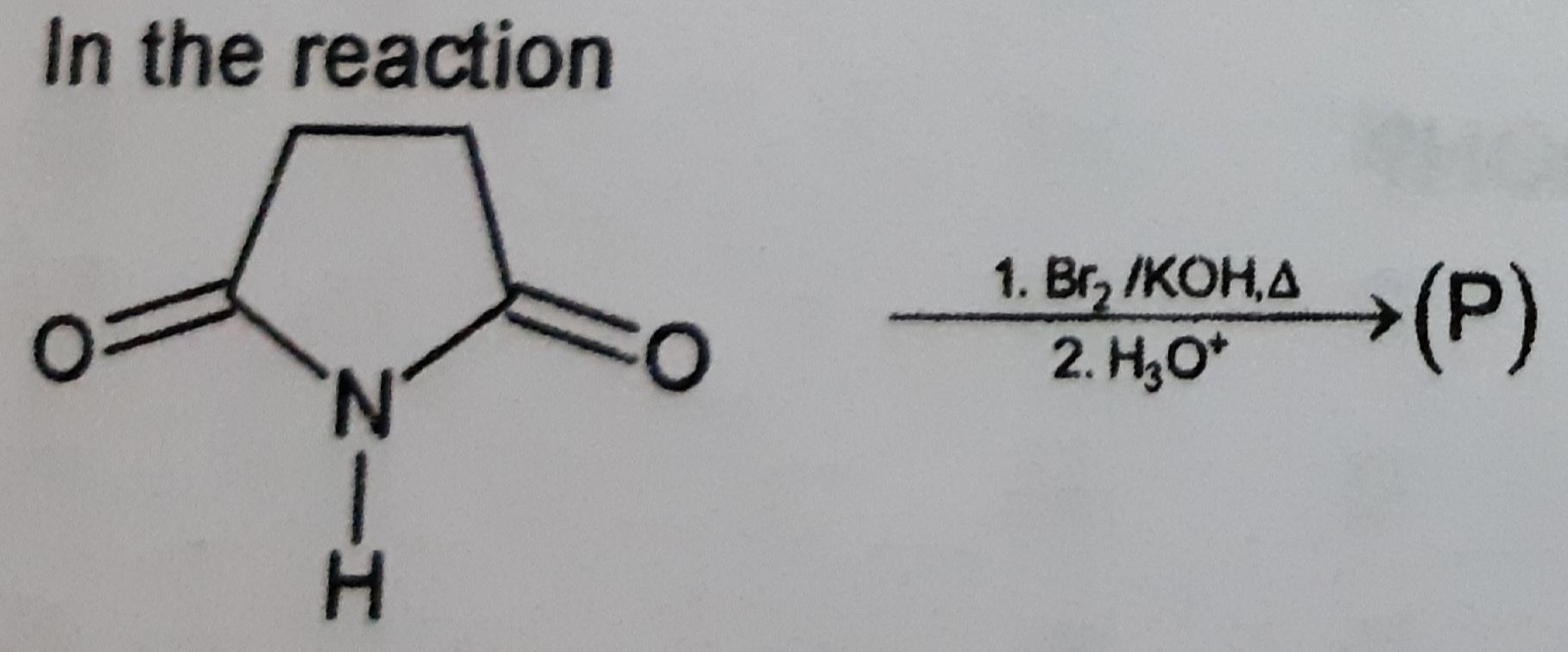

 Z is :-
(1)
CH3—CH2—OH
(2)
CH3—NH2
(3)
CH3—OH
(4)
CH3—CH2—NH2
Z is :-
(1)
CH3—CH2—OH
(2)
CH3—NH2
(3)
CH3—OH
(4)
CH3—CH2—NH2