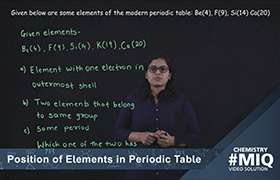
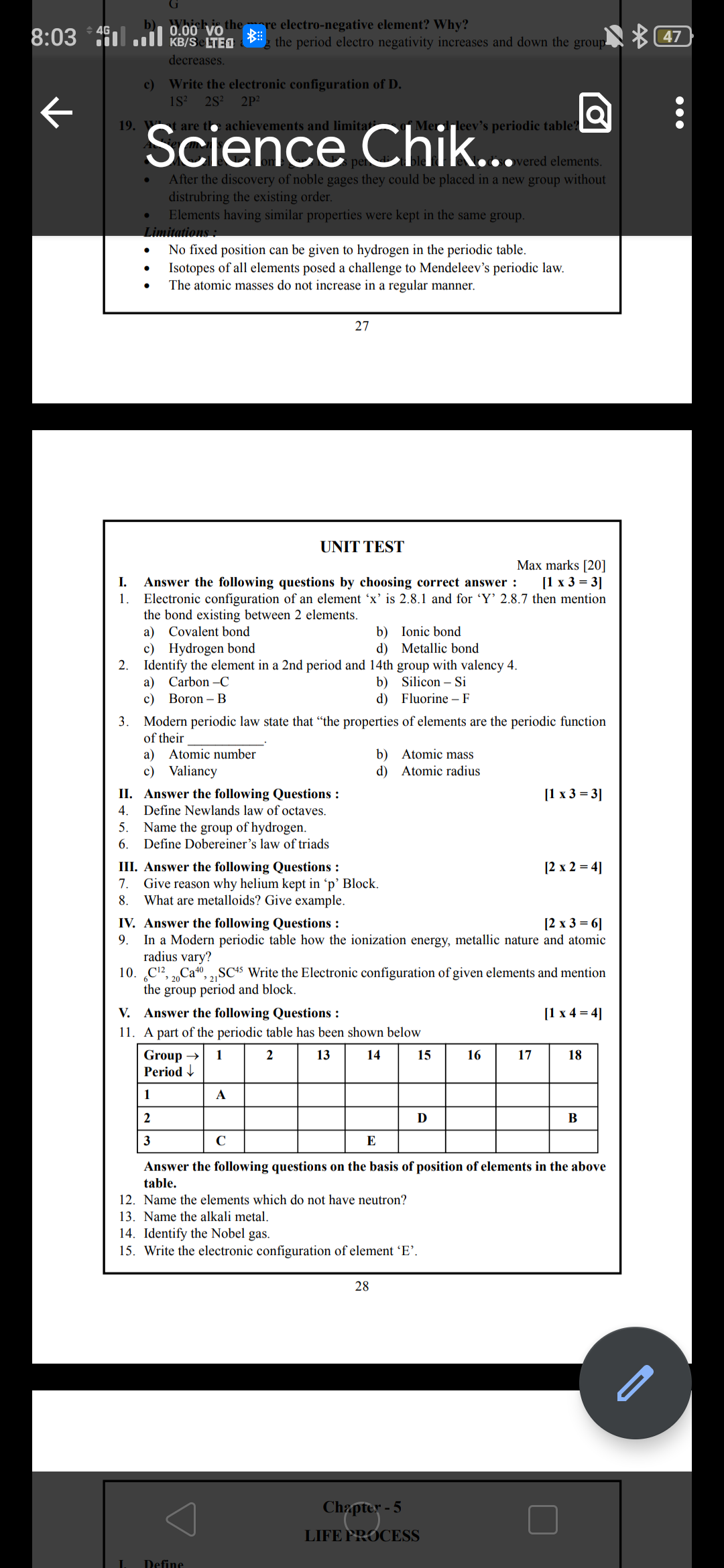
CBSE Class 10 Answered
Why the atomic volume of elements goes on decreasing as the atomic number increases in the third period?
Asked by tejasdd | 11 Sep, 2010, 11:53: AM
atomic radius decreases as we move from left to right
The distance from the nucleus to the outermost shell depends on the electrostatic attraction (nuclear charge) that the nucleus exerts on the electrons of the outer shell. More the nuclear charge closer are the shell and electrons, hence smaller is the atomic radius of an atom.
With the increase in the atomic number (increased number of protons, electrons and neutrons) in the 3rd period, the net positive charge of the nucleus gradually increases. This increased positive charge exerts a greater attraction on the shells and attract the electrons in the shells a little closer to the nucleus. Hence, sodium has the largest atom and chlorine the smallest
Answered by | 12 Sep, 2010, 09:48: AM
Application Videos
Concept Videos
CBSE 10 - Chemistry
Asked by dhumalchhaya13 | 26 May, 2022, 11:05: PM
CBSE 10 - Chemistry
Asked by kanchanbalpande82 | 12 Apr, 2022, 11:01: AM
CBSE 10 - Chemistry
Asked by kanchanbalpande82 | 12 Apr, 2022, 11:01: AM
CBSE 10 - Chemistry
Asked by waghmaresheetal78 | 27 Dec, 2021, 05:02: PM
CBSE 10 - Chemistry
Asked by ksheera36 | 03 Jun, 2021, 08:35: PM
CBSE 10 - Chemistry
Asked by vungtsaniyanthan | 16 May, 2021, 06:32: PM
CBSE 10 - Chemistry
Asked by sinhagopalakumara | 01 May, 2021, 08:16: PM
CBSE 10 - Chemistry
Asked by advssdrall | 26 Mar, 2021, 07:43: AM
CBSE 10 - Chemistry
Asked by kavithasenthil148 | 06 Feb, 2021, 07:09: PM
CBSE 10 - Chemistry
Asked by mina6poonam | 21 Jan, 2021, 04:57: PM