CBSE Class 12-science Answered
Why primary alchohols are more acidic than secondary and tertiary
Asked by singhdivyanshu633 | 08 Jul, 2015, 08:57: PM
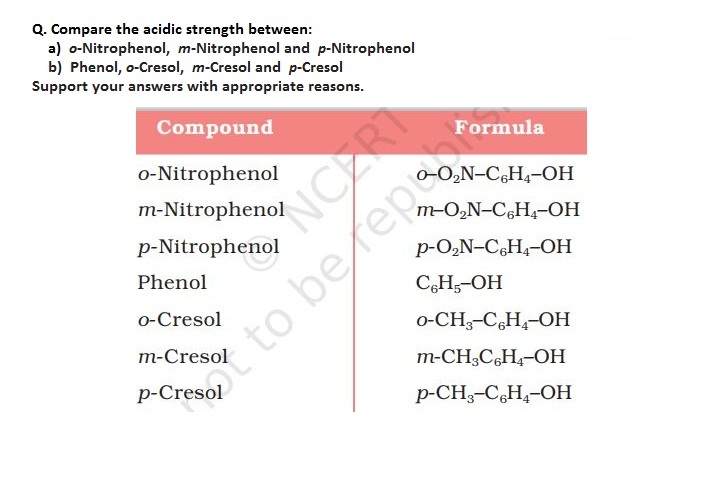
In case of alcohols acidic characters depends upon the O-H bond polarity. More the polar bond more acidic will be the respective alcohol. This polarity gets affected by electron donating group. If electron donating group increases polarity decreases.
The number of electron donating groups decreases in order:
Tertiary alcohol > Secondary alcohol > primary alcohol
Primary alcohols has more polar O-H bond than secondary alcohol and secondary alcohol has more polar O-H bond than tertiary alcohol.
Thus acidic strenght decreases in the following order:
Primary alcohol > secondary alcohol > tertiary alcohol
Answered by Arvind Diwale | 09 Jul, 2015, 11:33: AM
Application Videos
Concept Videos
CBSE 12-science - Chemistry
Asked by kavitabawane190 | 08 Mar, 2024, 05:24: PM
CBSE 12-science - Chemistry
Asked by rp0055293 | 07 Feb, 2024, 08:28: AM
CBSE 12-science - Chemistry
Asked by sanyaagrahari8888 | 28 Nov, 2023, 01:07: PM
CBSE 12-science - Chemistry
Asked by bithikapurkait09052005 | 24 Jun, 2022, 08:35: AM
CBSE 12-science - Chemistry
Asked by rohanm | 14 Feb, 2022, 04:49: PM
CBSE 12-science - Chemistry
Asked by vipulverma | 14 Feb, 2022, 04:44: PM
CBSE 12-science - Chemistry
Asked by kaziryan.05 | 23 Jun, 2021, 08:02: PM
CBSE 12-science - Chemistry
Asked by deepakatur454 | 28 Nov, 2020, 04:29: PM
CBSE 12-science - Chemistry
Asked by holebasubudihal | 09 Aug, 2020, 02:58: PM
CBSE 12-science - Chemistry
Asked by sulaikhasulu393 | 12 Jun, 2020, 09:34: PM