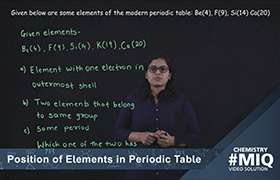
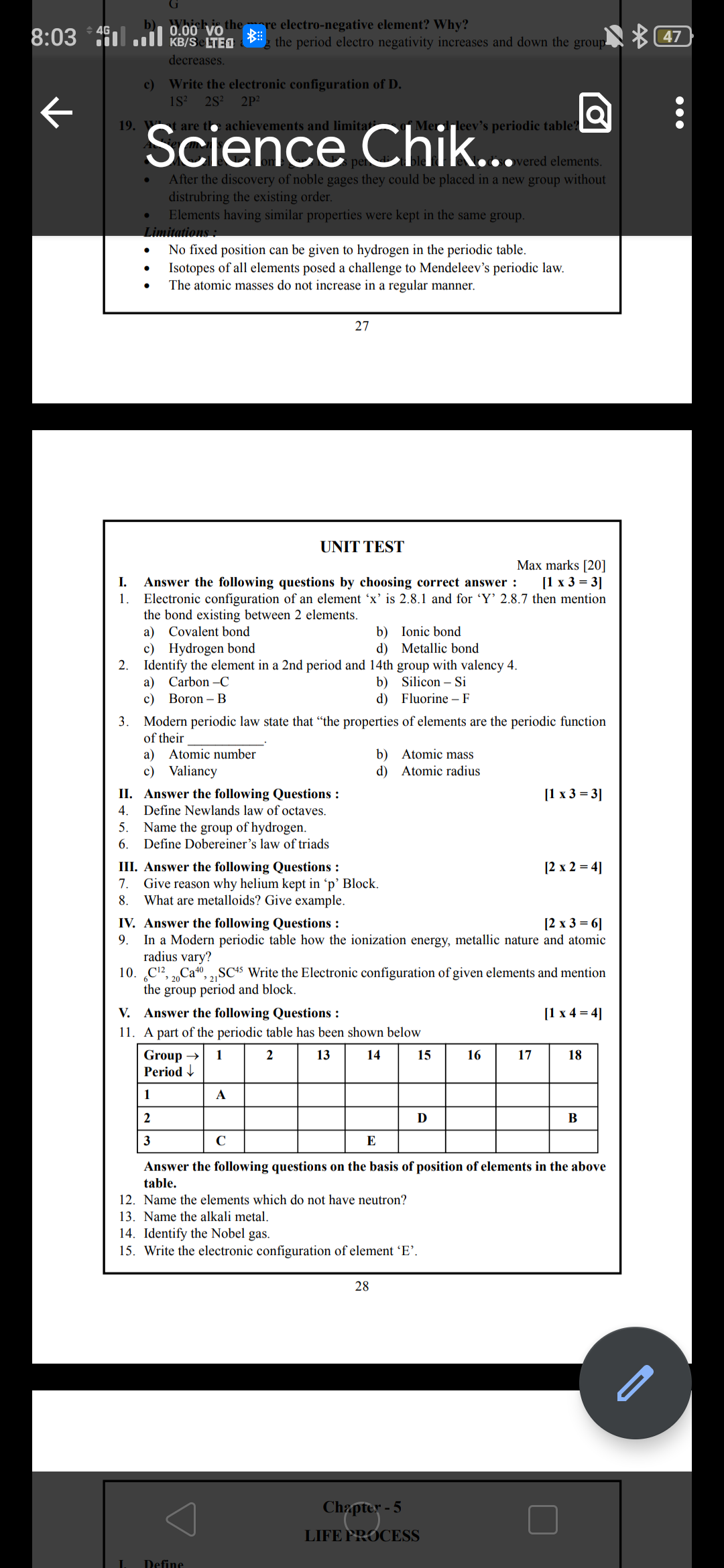
CBSE Class 10 Answered
Why only a specific one is depicted in the periodictable, among all other isotopes of an element ?
Asked by | 25 Jun, 2008, 01:43: PM
Modern periodic law states that atoms properties are a function of heir atomic numbers.So the atoms are places in periodic table based on their atomic numbers.Now as all the isotopes have same atomic number,they are all depicted singl on the periodic table by their atomic numbers.
Answered by | 04 Jul, 2008, 03:28: PM
Application Videos
Concept Videos
CBSE 10 - Chemistry
Asked by dhumalchhaya13 | 26 May, 2022, 11:05: PM
CBSE 10 - Chemistry
Asked by kanchanbalpande82 | 12 Apr, 2022, 11:01: AM
CBSE 10 - Chemistry
Asked by kanchanbalpande82 | 12 Apr, 2022, 11:01: AM
CBSE 10 - Chemistry
Asked by waghmaresheetal78 | 27 Dec, 2021, 05:02: PM
CBSE 10 - Chemistry
Asked by ksheera36 | 03 Jun, 2021, 08:35: PM
CBSE 10 - Chemistry
Asked by vungtsaniyanthan | 16 May, 2021, 06:32: PM
CBSE 10 - Chemistry
Asked by sinhagopalakumara | 01 May, 2021, 08:16: PM
CBSE 10 - Chemistry
Asked by advssdrall | 26 Mar, 2021, 07:43: AM
CBSE 10 - Chemistry
Asked by kavithasenthil148 | 06 Feb, 2021, 07:09: PM
CBSE 10 - Chemistry
Asked by mina6poonam | 21 Jan, 2021, 04:57: PM