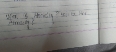JEE Class main Answered
Why is the dimensional formula of Boltzmann constant and gas constant same
Asked by harshpandeykp20 | 18 Aug, 2021, 08:31: AM
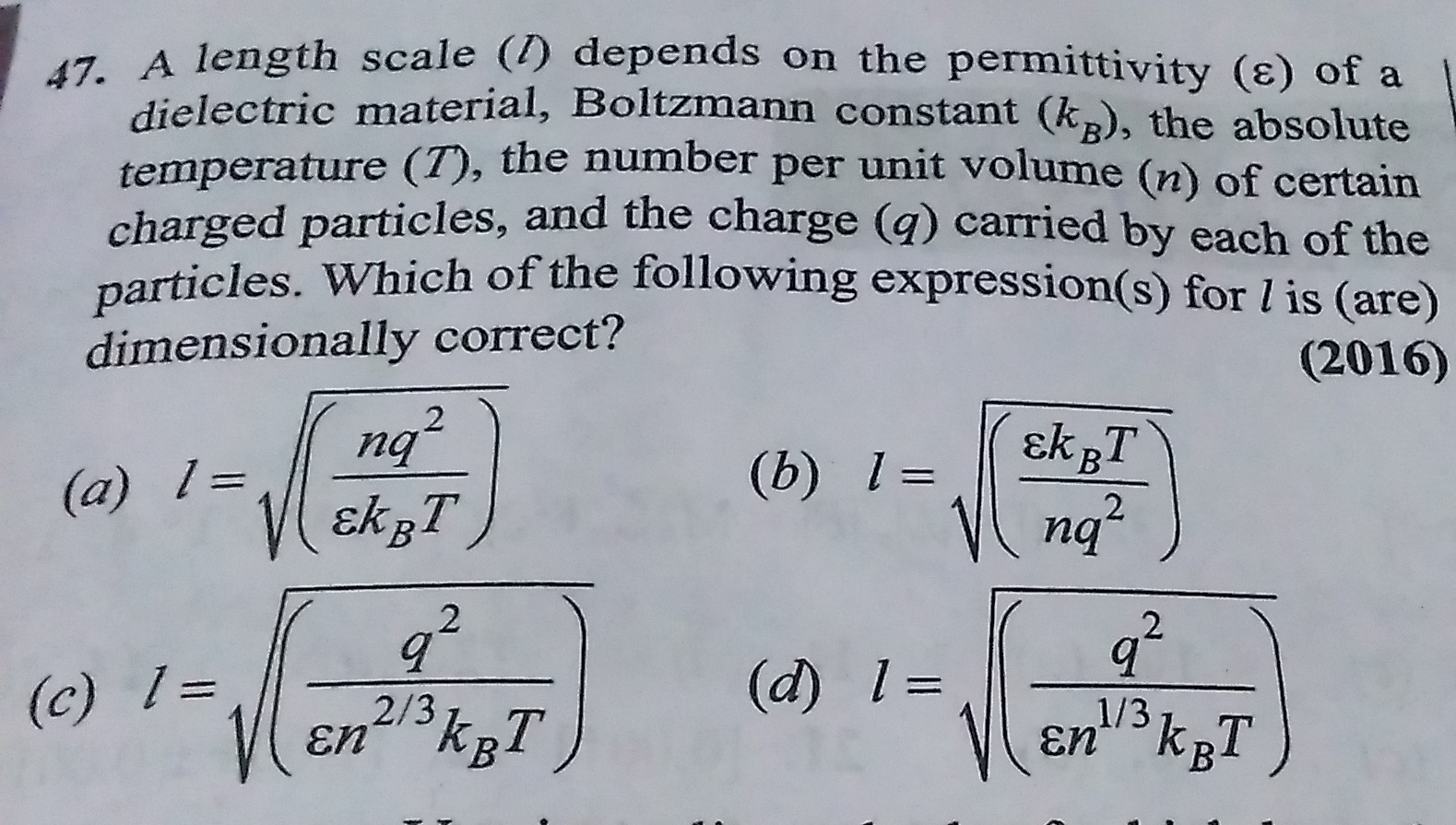
Boltzmann constant = Energy/ Temperature
= Energy x (Tempertaure)-1
Dimensions of Energy = [M1 L2 T -2]
Dimensions of Temperature = [K]
Thus,
Dimensions of Boltzmann constant = [M1 L2 T -2] x [K]-1 = [M1 L2 T -2 K-1]
Gas constant = Pressure x Volume / (Total no.of moles x Temperature)
= Pressure x Volume x (Total no.of moles x Temperature)-1
i.e
R = PV/nT
Dimensions of Pressure = [M1 L-1 T -2]
Dimensions of Volume = [L3]
Dimensions of temperature = [K]
Dimensions of Gas constant = [M1 L-1 T -2] x [L3] x [K]-1 = [M1 L2 T -2 K-1]
Thus, you can observe that both Boltzmann constant and Gas constant have same dimensional formula
Answered by Shiwani Sawant | 18 Aug, 2021, 17:41: PM
JEE main - Physics
Asked by nidhikalluthattu | 11 Jul, 2024, 20:00: PM
JEE main - Physics
Asked by rohanhi348 | 14 Jun, 2024, 20:44: PM
JEE main - Physics
Asked by lalamsurya41 | 27 May, 2024, 10:37: AM
JEE main - Physics
Asked by avhadbalaji2 | 24 Jan, 2024, 14:12: PM
JEE main - Physics
Asked by kartikchauhan2425 | 13 Jan, 2024, 17:37: PM
JEE main - Physics
Asked by mvijithareddy67 | 13 Jan, 2024, 09:26: AM
JEE main - Physics
Asked by mages032006 | 26 Jun, 2023, 18:37: PM
JEE main - Physics
Asked by mahadhasyamvarshini | 05 Feb, 2023, 22:25: PM
JEE main - Physics
Asked by harshpandeykp20 | 18 Aug, 2021, 08:31: AM