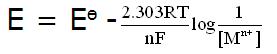CBSE Class 12-science Answered
Why is the value of the E of a galvanic cell less than expected?
Asked by uma.therighter | 23 Jan, 2010, 09:40: PM
See Eocell = Eocathode - Eoanode
This is the emf of the cell when no current is drawn through the cell. The conditions are standard conditions i.e. the temperature is 298 K and the concentration of the solutions is unity..
But when the concentration of the solutions change the cell potential decreases.
Therefore, nernst equation is followed under such conditions:
Answered by | 25 Jan, 2010, 09:56: AM
Concept Videos
CBSE 12-science - Chemistry
Asked by yashwanthgowdakn4 | 22 Feb, 2024, 09:14: PM
CBSE 12-science - Chemistry
Asked by sy985326 | 21 Feb, 2024, 04:23: AM
CBSE 12-science - Chemistry
Asked by rishitadekaraja123 | 07 Feb, 2024, 08:41: AM
CBSE 12-science - Chemistry
Asked by summiafroz31 | 06 Feb, 2024, 08:39: PM
CBSE 12-science - Chemistry
Asked by skmdsajid04 | 14 Jan, 2024, 09:23: AM
CBSE 12-science - Chemistry
Asked by samskruthikrishn | 12 Jan, 2024, 10:11: AM
CBSE 12-science - Chemistry
Asked by aryamankrsinha2002 | 29 Nov, 2023, 11:39: AM
CBSE 12-science - Chemistry
Asked by 2507king2006 | 03 Oct, 2023, 07:12: AM
CBSE 12-science - Chemistry
Asked by keerthana.d.cst.2022 | 22 Aug, 2023, 08:18: PM
CBSE 12-science - Chemistry
Asked by banneramadevi | 26 Jul, 2023, 08:51: PM