CBSE Class 11-science Answered
Why is pressure an intensive property? Please explain it according to force per unit area with examples.
Asked by Varsneya Srinivas | 13 Oct, 2016, 10:37: AM
Intensive property:
These are those properties which depend only upon the nature of the substance and are independent of the amount of the substance present in the system.
Ratio of two extensive properties is an intensive property.
Now,
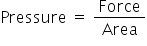
Here, force and area both are extensive properties. Ratio of these two extensive properties is an intensive prooperty which is pressure.
Answered by Prachi Sawant | 13 Oct, 2016, 11:53: AM
CBSE 11-science - Chemistry
Asked by rukayabatool395 | 24 Mar, 2024, 07:48: PM
CBSE 11-science - Chemistry
Asked by rama26516 | 12 Mar, 2022, 01:49: PM
CBSE 11-science - Chemistry
Asked by mossewalasindhu | 20 Nov, 2021, 02:16: PM
CBSE 11-science - Chemistry
Asked by chandankumargochhayat6 | 19 May, 2021, 08:48: AM
CBSE 11-science - Chemistry
Asked by saimahima2005 | 16 Jul, 2020, 06:28: PM
CBSE 11-science - Chemistry
Asked by Leenatripathi20 | 10 Feb, 2020, 07:12: PM
CBSE 11-science - Chemistry
Asked by ABHILASHA | 26 Aug, 2019, 11:41: PM
CBSE 11-science - Chemistry
Asked by hritikch37 | 16 Dec, 2018, 10:09: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 15 Jun, 2016, 02:53: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 13 Aug, 2014, 11:18: AM

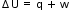 .
.