CBSE Class 10 Answered
Why is it that weak acids and weak bases do not completely ionize when dissolved in water while strong acids and strong bases do? Please explain the reason in detail
Asked by kumar.ashlesha | 18 Jun, 2015, 04:52: PM
In case of weak acid some molecules are dissociating into ions; some ions are recombining to form molecules. Due to which in the ionization of a weak acid there are fewer ions than molecules.
For example: Ionisation of acetic acid,
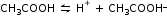
In case of strong acid there is no recombination so we can observe complete ionisation.
For example: Ionisation of nitric acid,
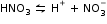
Answered by Arvind Diwale | 19 Jun, 2015, 12:10: PM
Application Videos
Concept Videos
CBSE 10 - Chemistry
Asked by aggrwalmontek | 14 Sep, 2023, 10:43: PM
CBSE 10 - Chemistry
Asked by manisha.5154 | 15 Jun, 2022, 02:52: PM
CBSE 10 - Chemistry
Asked by ranishoba947 | 10 May, 2022, 09:04: PM
CBSE 10 - Chemistry
Asked by Trisha Gupta | 23 Nov, 2021, 12:29: AM
CBSE 10 - Chemistry
Asked by anshika.dubey9809 | 11 Nov, 2021, 07:42: PM
CBSE 10 - Chemistry
Asked by bhavikabhatia1125 | 10 Jul, 2021, 10:27: PM
CBSE 10 - Chemistry
Asked by palakkothari46 | 22 Jun, 2021, 11:58: AM
CBSE 10 - Chemistry
Asked by nitikakaliramana466 | 14 May, 2021, 09:37: AM
CBSE 10 - Chemistry
Asked by ayan1.chatterjee | 07 May, 2021, 08:05: PM










