CBSE Class 11-science Answered
Why is compressibility factor of Hydrogen and Helium greter than 1?
Asked by Ankit Jatiya | 30 Sep, 2014, 09:22: AM
Dear ankit.jatiya715@gmail.com
Thanks for asking us a question in Ask the Expert section of TopperLearning.com.
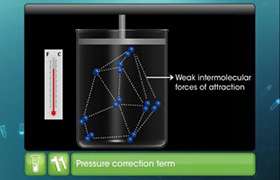
This is observed because the gases like hydrogen and helium shows intermolecular repulsive forces which cause the actual volumes to be greater than the ideal values.
Regards
Topperlearning Team.
Answered by Arvind Diwale | 30 Sep, 2014, 11:23: AM
Concept Videos
CBSE 11-science - Chemistry
Asked by khansuhana410 | 22 Dec, 2022, 11:48: AM
CBSE 11-science - Chemistry
Asked by aiqbal86592 | 28 May, 2019, 12:20: PM
CBSE 11-science - Chemistry
Asked by lovemaan5500 | 26 Jan, 2019, 12:57: PM
CBSE 11-science - Chemistry
Asked by nasankalagi04 | 17 Oct, 2018, 08:47: PM
CBSE 11-science - Chemistry
Asked by smanishkumar2002 | 04 Aug, 2018, 05:38: AM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 17 Jun, 2016, 12:02: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 21 Apr, 2015, 12:19: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 21 Apr, 2015, 12:21: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 21 Apr, 2015, 12:31: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 17 Jun, 2016, 12:01: PM