CBSE Class 12-science Answered
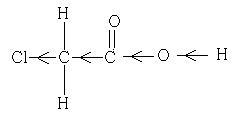
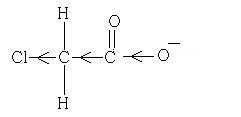
Why is Chloroethanoic acid about 100 times stronger than acetic acid ?
Asked by pratikbharadia | 28 Dec, 2009, 12:05: PM
Since Chloro group is an electron withdrawing group. It has -I effect.
Chloro group withdraws the electron density fronm the O-H bond. Thus the release of H+ is easier and hence increase in acidity.
Also, since chloro group is an electron withdrawing group, it increase the acidity of carboxylic acids by stabilising the conjugate base through delocalisation of the negative charge by electron withdrawing inductive effect.
Answered by | 28 Dec, 2009, 12:36: PM
Concept Videos
CBSE 12-science - Chemistry
Asked by ukg8612 | 15 Apr, 2024, 07:36: PM
CBSE 12-science - Chemistry
Asked by ajayarchi | 08 Feb, 2024, 03:43: AM
CBSE 12-science - Chemistry
Asked by pallasriramulu9 | 24 Dec, 2023, 06:05: AM
CBSE 12-science - Chemistry
Asked by bsaheliya | 22 Dec, 2023, 09:53: PM
CBSE 12-science - Chemistry
Asked by ygarg8323 | 18 Apr, 2022, 12:47: PM
CBSE 12-science - Chemistry
Asked by kaziryan.05 | 30 Jun, 2021, 04:52: PM
CBSE 12-science - Chemistry
Asked by kaziryan.05 | 29 Jun, 2021, 08:36: AM
CBSE 12-science - Chemistry
Asked by kaziryan.05 | 28 Jun, 2021, 02:34: PM
CBSE 12-science - Chemistry
Asked by saimerala007 | 22 May, 2021, 02:08: PM
CBSE 12-science - Chemistry
Asked by amitkumar.cis | 31 Dec, 2020, 10:45: AM