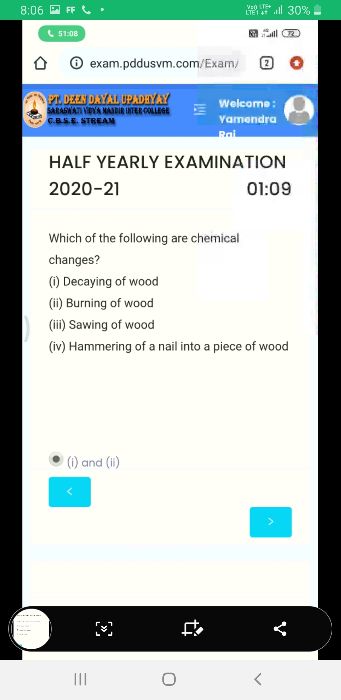
CBSE Class 9 Answered
Why hot water freezes than cold water??
Asked by Anishkjainacus | 03 Sep, 2018, 08:07: AM
- The most simple fact that freezing is an event when a body loses its own heat to the surrounding.
- In standard temperature, pressure and humidity conditions, it takes lesser time for a hot body to lose its heat to the surrounding when compared to the process of a cold body losing its heat energy to the surrounding.
- The heat energy always flows from a hot body to the comparitively colder surroundings /body.
- That is why in normal conditions, it is easier and quicker for a hot body to freeze when compared to the process of freezing the already colder body.
Answered by Abhijeet Mishra | 03 Sep, 2018, 10:49: AM
Application Videos
Concept Videos
CBSE 9 - Chemistry
Asked by rubinapathan228 | 29 Jun, 2023, 05:45: PM
CBSE 9 - Chemistry
Asked by yforyt3672 | 09 Apr, 2023, 07:37: PM
CBSE 9 - Chemistry
Asked by pranavtamboli65.9 | 02 Aug, 2022, 08:12: PM
CBSE 9 - Chemistry
Asked by ritapriya126 | 20 Apr, 2022, 04:01: PM
CBSE 9 - Chemistry
Asked by renukaramaswamyr | 08 Sep, 2021, 12:32: PM
CBSE 9 - Chemistry
Asked by gpranithasri | 27 Jul, 2021, 01:23: PM
CBSE 9 - Chemistry
Asked by suhanivasi007 | 04 May, 2021, 05:52: PM
CBSE 9 - Chemistry
Asked by pk36229905 | 06 Apr, 2021, 07:38: AM
CBSE 9 - Chemistry
Asked by anubhavev | 30 Oct, 2020, 06:35: PM