CBSE Class 9 Answered
Why gases exert pressure
Asked by Sumaks333 | 01 Jul, 2018, 12:21: PM
According to the kinetic theory of gases, gas particles are in continuous motion, hence randomly they colloids on each other and also the walls of the container with force.
Due to this collision gas particles exert pressure.
Answered by Ramandeep | 02 Jul, 2018, 12:33: PM
Application Videos
Concept Videos
CBSE 9 - Chemistry
Asked by rubinapathan228 | 29 Jun, 2023, 05:45: PM
CBSE 9 - Chemistry
Asked by yforyt3672 | 09 Apr, 2023, 07:37: PM
CBSE 9 - Chemistry
Asked by pranavtamboli65.9 | 02 Aug, 2022, 08:12: PM
CBSE 9 - Chemistry
Asked by ritapriya126 | 20 Apr, 2022, 04:01: PM
CBSE 9 - Chemistry
Asked by renukaramaswamyr | 08 Sep, 2021, 12:32: PM
CBSE 9 - Chemistry
Asked by gpranithasri | 27 Jul, 2021, 01:23: PM
CBSE 9 - Chemistry
Asked by suhanivasi007 | 04 May, 2021, 05:52: PM
CBSE 9 - Chemistry
Asked by pk36229905 | 06 Apr, 2021, 07:38: AM
CBSE 9 - Chemistry
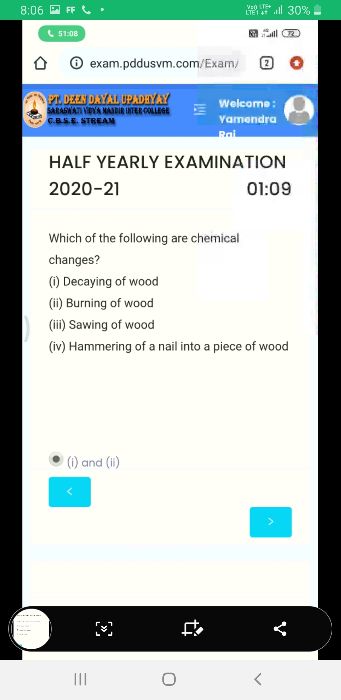
Asked by anubhavev | 30 Oct, 2020, 06:35: PM