ICSE Class 8 Answered
Why does the sodium sulphate solution and barium chloride solution reacts as white solution.
Asked by arushsoni16.8spicertl | 14 May, 2020, 11:25: AM
When an aqueous solution of sodium sulphate reacts with an aqueous solution of barium chloride, insoluble barium sulphate along with solution of sodium chloride is formed. If the reactants are in solid state, then reaction will not take place between sodium sulphate and barium chloride.
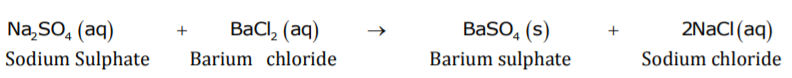 Reaction between aqueous solution of sodium sulphate and aqueous solution of barium chloride is a double displacement reaction.
Reaction between aqueous solution of sodium sulphate and aqueous solution of barium chloride is a double displacement reaction.
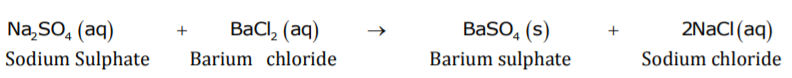
Answered by Ramandeep | 14 May, 2020, 12:25: PM
Concept Videos
ICSE 8 - Chemistry
Asked by n.tara345 | 16 Aug, 2023, 06:50: PM
ICSE 8 - Chemistry
Asked by nihalcrj2006 | 27 Jan, 2021, 11:37: AM
ICSE 8 - Chemistry
Asked by mishragayatri483 | 27 Dec, 2020, 06:11: PM
ICSE 8 - Chemistry
Asked by sp_colney | 01 Aug, 2020, 04:14: PM
ICSE 8 - Chemistry
Asked by manishpatil37.8spicertl | 29 Jun, 2020, 11:14: AM
ICSE 8 - Chemistry
Asked by arushsoni16.8spicertl | 14 May, 2020, 11:25: AM
ICSE 8 - Chemistry
Asked by tirthabasibagh77 | 16 Feb, 2020, 07:01: PM
ICSE 8 - Chemistry
Asked by Nidhimalhotra888 | 06 Dec, 2019, 04:31: PM
ICSE 8 - Chemistry
Asked by Sandeephundal574 | 16 May, 2019, 09:56: PM
ICSE 8 - Chemistry
Asked by divyanshchaubey | 17 Feb, 2019, 11:19: AM




